4 Minutes
Understanding Parkinson's Disease and Its Global Challenge
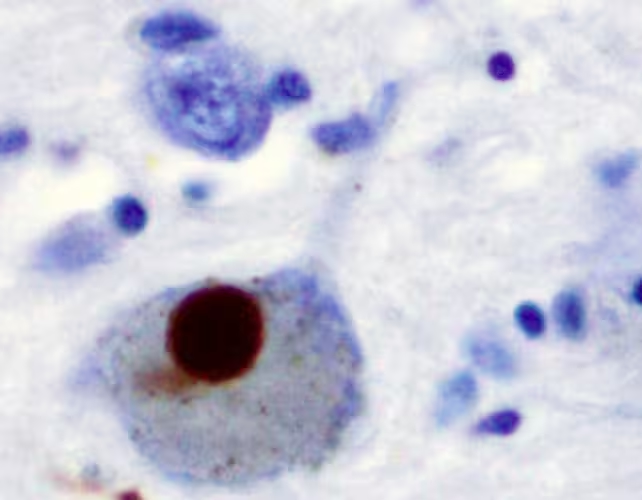
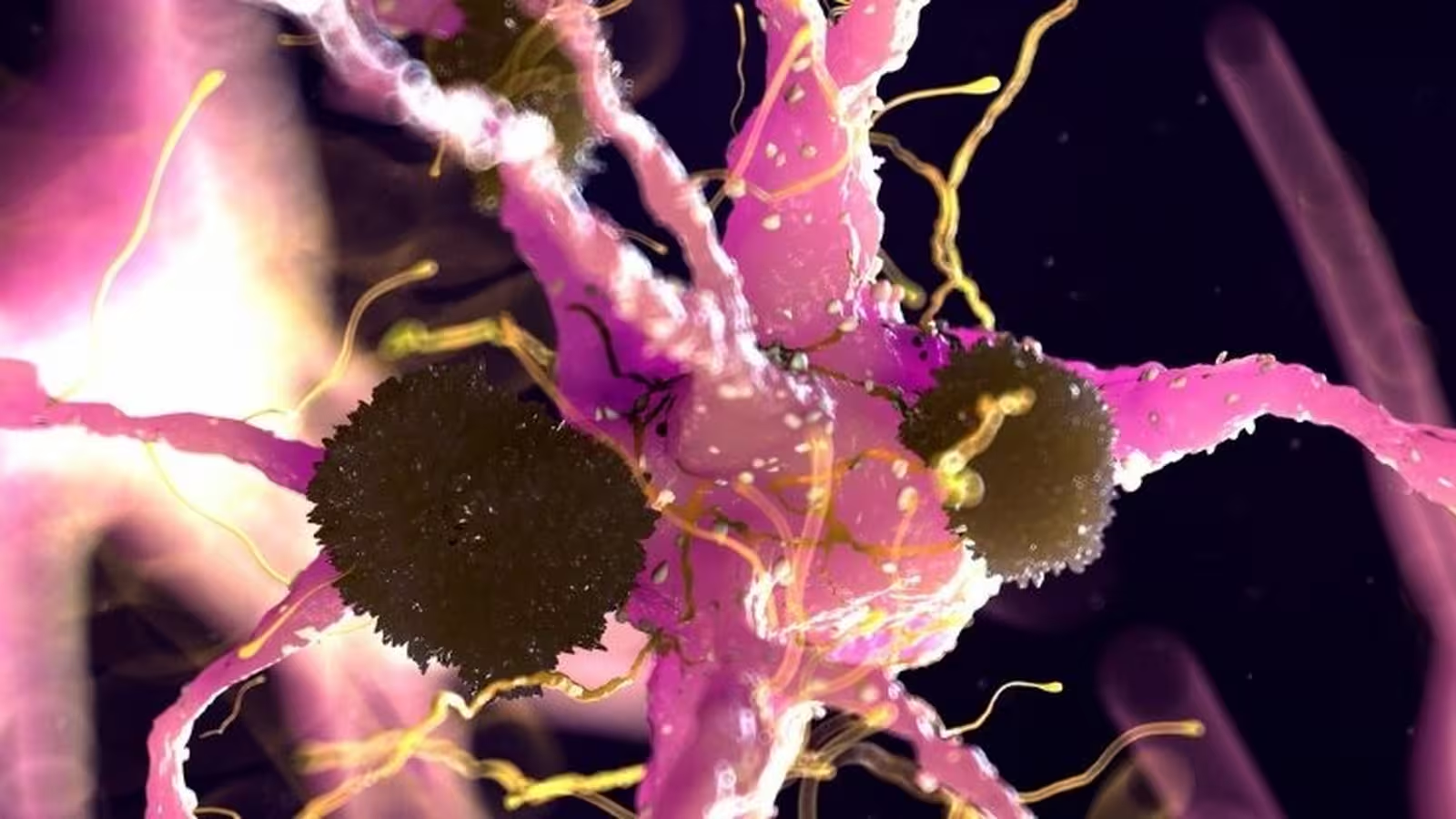
Parkinson's disease remains one of the most prevalent neurodegenerative conditions in the world, affecting over 8.5 million people. Characterized by progressive symptoms such as tremors, muscle rigidity, balance problems, speech difficulties, sleep disruptions, and mental health challenges, its burden is only anticipated to rise as populations age. It currently stands as the second most common neurodegenerative disorder, surpassed only by Alzheimer's disease. As the disease advances, patients may lose their ability to walk or speak, predominantly due to the loss or dysfunction of dopamine-producing neurons within a brain region known as the substantia nigra. A key pathological hallmark is the accumulation of Lewy bodies—clumps of misfolded proteins called alpha-synuclein—that disrupt neural function as they travel from cell to cell.
The Role of Alpha-Synuclein, Aplp1, and Lag3 in Parkinson's Progression
Alpha-synuclein serves an important function in aiding communication between neurons under normal conditions. However, when misfolded, it forms insoluble aggregations that are believed to drive the neurodegenerative process in Parkinson's disease. New research published by an international consortium of scientists has illuminated the critical roles two proteins, Aplp1 and Lag3, play in the spread of these toxic alpha-synuclein clumps within the brain.
In prior animal studies, the protein Lag3 was shown to bind to alpha-synuclein and facilitate its pathological spread through brain tissue. However, removing Lag3 alone did not entirely halt this process, indicating that additional proteins were involved. Neuroscientists, including Valina Dawson and Xiaobo Mao from Johns Hopkins University, turned their focus to Aplp1, a surface protein also present in neural cells. Using genetically engineered mouse models lacking either Aplp1 or Lag3—or both—the researchers discovered that each protein can independently mediate the uptake of misfolded alpha-synuclein by neurons. More strikingly, when both proteins were absent, there was a 90% reduction in the internalization of toxic alpha-synuclein, underscoring their combined importance in disease propagation.
An Existing FDA-Approved Drug Shows Promise in Blocking Parkinson's Mechanisms
Perhaps the most significant finding from this study concerns a drug already approved by the U.S. Food and Drug Administration for cancer treatment: nivolumab/relatlimab, a medication prescribed for melanoma. This drug contains an antibody that specifically targets Lag3. When administered to mice, the Lag3 antibody therapy disrupted the interaction between Lag3 and Aplp1, effectively blocking the majority of alpha-synuclein's entry into neurons and sharply reducing the formation of disease-causing protein aggregates.
"Now that we know how Aplp1 and Lag3 interact, we have a new way of understanding how alpha-synuclein contributes to the disease progression of Parkinson's disease," Dr. Xiaobo Mao explained. The research revealed that blocking this interaction offers a more pronounced protective effect than targeting Lag3 alone, due to the close association between the two proteins.
"The anti-Lag3 antibody was successful in preventing further spread of alpha-synuclein seeds in the mouse models and exhibited better efficacy than Lag3-depletion because of Aplp1's close association with Lag3," commented Dr. Ted Dawson, further emphasizing the translational promise of the therapy.
Implications for Future Therapies and Next Steps
This breakthrough not only unravels a crucial mechanism driving Parkinson’s progression, but also suggests that drugs already in clinical use for other conditions like melanoma could be rapidly repurposed as neurodegenerative disease treatments. The next phase for this research involves testing Lag3 antibody therapy in animal models of Parkinson's and Alzheimer's disease, as initial findings suggest a parallel role for Lag3 in both disorders. If successful, these studies could pave the way for new clinical trials, offering hope for slowing or halting the advance of Parkinson's and related neurodegenerative diseases.
Conclusion
These discoveries mark a significant step forward in Parkinson’s disease research, highlighting how understanding the interaction between Aplp1 and Lag3 opens up new therapeutic avenues. The potential to repurpose an FDA-approved drug could accelerate the development of the first effective treatments for Parkinson’s, revolutionizing care for millions worldwide and shedding light on the disease mechanisms that underlie many forms of neurodegeneration.
Source: link.springer


Comments