6 Minutes
The Essential Question: Where Do Atoms Come From?
The great physicist Richard Feynman once declared that the most profound scientific insight is that all matter is made of atoms. These tiny building blocks are the foundation of everything around us, from the air we breathe to the furthest galaxies. But where do atoms originate, and how did they come to populate the universe?
Understanding atomic formation is a cornerstone of modern physics and cosmology. Despite decades of research, scientists are still piecing together the events that led to the birth of atoms, drawing on knowledge from nuclear physics, quantum mechanics, and cosmological observations. Their findings reveal a dramatic story stretching back to the very first moments after the Big Bang.
What Are Atoms? The Structure of Matter
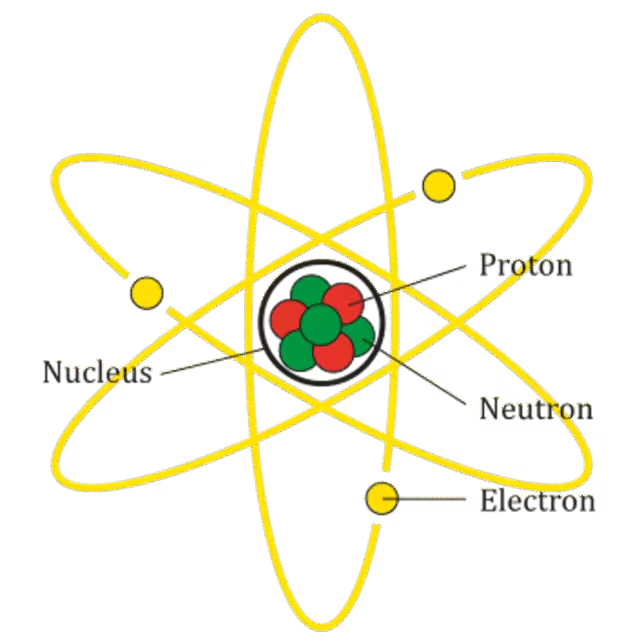
Atoms are the smallest units of ordinary matter that retain the properties of an element. Each atom consists of a central nucleus composed of positively charged protons and electrically neutral neutrons. Orbiting this nucleus are electrons, particles with a negative charge. The number of protons defines the chemical element; for instance, hydrogen atoms have one proton, while helium atoms have two.
Atoms are overall electrically neutral because they possess equal numbers of protons and electrons. While thousands of different atoms exist, hydrogen and helium are by far the most abundant in the cosmos. On Earth, atoms such as carbon, oxygen, and nitrogen also play key roles in forming the diverse molecules essential for life.
Scientists refer to all atoms with the same number of protons as an "element." This classification underpins the periodic table, which organizes all known chemical elements and their atomic structures.
The Birth of the First Atoms After the Big Bang
The universe began with the Big Bang roughly 13.8 billion years ago, erupting as an incredibly hot and dense state. In the earliest moments, temperatures and energies were far too extreme for atoms to exist; matter was a chaotic soup of unbound protons, neutrons, electrons, and photons.
About 400,000 years after the Big Bang—a relatively brief time on cosmic scales—the universe cooled enough for electrons to slow down, allowing them to be captured by atomic nuclei. This event, known in cosmology as "recombination," marked the era when hydrogen and helium atoms formed in vast quantities. At this point, the universe was about one-thousandth its present size and had a temperature near 2,760 degrees Celsius (5,000 degrees Fahrenheit).
Prior to recombination, electrons' high energies prevented them from being bound to nuclei. Only as the universe expanded and cooled did the energy levels drop, enabling stable atoms to form. Helium and a heavier hydrogen isotope, deuterium, actually began forming just minutes after the Big Bang, when the universe’s temperature was over 556 million degrees Celsius (1 billion degrees Fahrenheit). Such intense environments were necessary for protons and neutrons to overcome their mutual repulsion and stick together, forming the first atomic nuclei.
Today, approximately 90% of the universe's ordinary matter consists of hydrogen atoms, with nearly 8% made up of helium—testifying to the importance of these ancient processes.
Forging Heavier Elements: The Role of Stars and Supernovas
While recombination birthed most of the universe’s hydrogen and helium, it did not produce heavier atoms like carbon, oxygen, or iron—elements essential for planets and life. These heavier elements could only be forged under far more extreme conditions, found within the hearts of massive stars.
In the intense pressures and temperatures of stellar cores—far hotter than our Sun—nuclear fusion reactions combine lighter atomic nuclei to form heavier ones. For example, stars several times the mass of the Sun reach internal temperatures exceeding 1 billion degrees Fahrenheit (556 million degrees Celsius), powerful enough to fuse protons and neutrons into heavier nuclei. This is only possible because of a fundamental force known as the strong nuclear force—which allows these particles to bind once close enough, despite the electromagnetic repulsion that would otherwise push positively charged protons apart.
The fusion processes in stars build elements up to iron on the periodic table. However, creating atoms heavier than iron (like gold, platinum, and uranium) requires even more energy than what fusion in regular stars can provide, as these heavy nuclei are more prone to splitting apart.

Supernovas: Cosmic Forges of Heavy Elements
When massive stars exhaust their nuclear fuel, they undergo catastrophic collapses known as supernovas. In these titanic explosions, the core of the star contracts rapidly, triggering a powerful release of energy. This environment is perfect for forming heavy elements—as neutrons and protons smashed together in the chaos generate nuclei heavier than iron. The explosion then scatters these newly formed atoms into space, where they eventually become part of new stars, planets, and, ultimately, living systems.
Beyond Supernovae: Neutron Star Mergers and Stellar Alchemy
Astrophysicists have detected other extraordinary processes that create even heavier elements. For example, when two neutron stars—ultra-dense remnants of dead stars—collide, immense energies are unleashed. These rare mergers generate gravitational waves and scatter vast amounts of gold and other heavy elements across the cosmos.
Scientists continue to explore the physics behind these events, using advanced telescopes, particle accelerators, and space missions to refine our understanding of cosmic nucleosynthesis.

The Unsolved Mystery: Dark Matter and the Limits of Atomic Theory
Even as researchers unravel the atomic origins of ordinary matter, a major puzzle remains. Observations suggest that about 85% of all matter in the universe is not made up of known atoms, but instead consists of a mysterious substance called dark matter. Unlike atoms, dark matter does not emit, absorb, or reflect light, making it invisible to traditional telescopes.
Scientists are actively searching for evidence of dark matter's composition, with experiments ranging from underground detectors to observations of distant galaxies and gravitational lensing. Solving the dark matter puzzle could further transform our understanding of the universe’s fundamental building blocks.
Conclusion
The creation of atoms—the tangible essence of matter—is one of the universe's most remarkable stories. From the universe’s searing infancy after the Big Bang, where hydrogen and helium formed as the first stable atoms, to the cosmic cauldrons of stars and the cataclysmic blasts of supernovas that forge heavier elements, the journey of atoms is also the journey of all material things. Unraveling how atoms form connects the vast scales of cosmology with the microscopic mysteries of quantum physics, while opening new windows to phenomena such as dark matter. As science advances, our cosmic origin story continues to unfold, offering deeper insight into the fundamental structure and evolution of the universe.
Source: theconversation



Comments