3 Minutes
Regulatory Milestone: Human Trials Approved for Groundbreaking SCI Therapy
In a historic development for regenerative medicine, both the U.S. Food and Drug Administration (FDA) and China's National Medical Products Administration (NMPA) have granted approval for the world's first clinical trial of an innovative spinal cord injury (SCI) treatment. This landmark decision marks a significant advancement for the over 15 million people globally who live with spinal cord injuries—many of whom face lifelong paralysis or severe disability due to the absence of effective cures.
The Science Behind the New Spinal Cord Injury Treatment
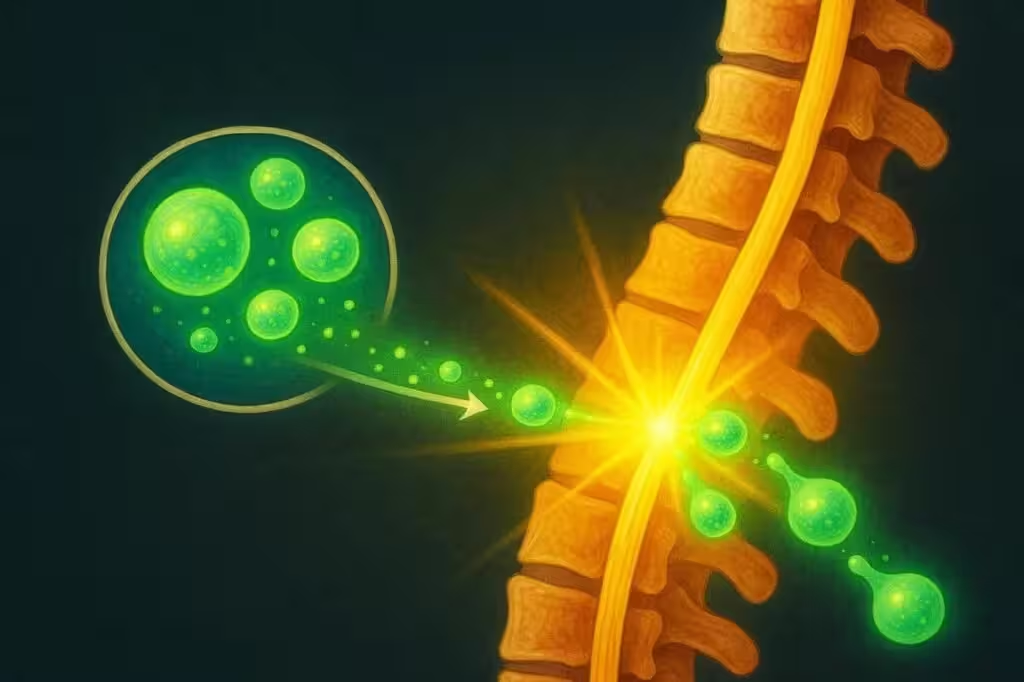
The approved therapy, developed by Chinese biotech firm XellSmart, leverages allogeneic induced pluripotent stem cells (iPSCs). Unlike traditional stem cell therapies that may require harvesting a patient’s own cells, this approach utilizes donor cells, allowing for a standardized, "off-the-shelf" treatment designed for broad applicability. The iPSC technology reprograms adult cells back into a pluripotent stem state, enabling them to differentiate into specialized cells capable of regenerating damaged spinal tissue.
Preclinical Progress and Next Steps
XellSmart’s therapy has undergone more than four years of rigorous research and preclinical development. The recent green light from both the FDA and NMPA authorizes the initiation of Phase I clinical trials, where the primary objectives will be to assess the safety, optimal dosing, and preliminary efficacy in human participants.
Why This Breakthrough Matters
SCI can result from various causes, including motor vehicle accidents, sports injuries, and falls from significant heights. Current treatment options focus mainly on acute care, surgery, and rehabilitation, which typically only provide limited improvement. The new allogeneic stem cell therapy stands out because it does not require cell extraction from patients, reducing complexity and making it a potentially scalable "universal" solution. Furthermore, the risk of immune rejection appears to be minimal, as allogenic iPSCs can be engineered to lower immunogenicity.
If the Phase I trial is successful, the research team plans to move rapidly to Phase II—potentially by 2028—expanding testing to a larger patient population and gathering more robust data on efficacy. The long-term goal is to make the treatment widely available within 5 to 7 years, offering new hope for millions living with spinal cord injury worldwide.
Conclusion
The pioneering approval of XellSmart’s iPSC-based spinal cord injury therapy for human trials represents a promising leap forward in regenerative medicine and neuroscience. While further testing is needed, this therapeutic strategy could transform the future of spinal cord injury treatment, offering the possibility of functional recovery where none existed before.

Comments