4 Minutes
Unlocking a New Approach to Alzheimer's and Dementia Treatment
Innovative scientific research is paving the way for fresh strategies in the fight against Alzheimer's disease and age-related cognitive decline. A landmark study conducted by scientists at the Federal University of Rio de Janeiro (UFRJ) and the University of São Paulo in Brazil has identified the protein hevin (also known as SPARCL-1) as a key player in preventing memory loss and supporting brain health in mice — a development with significant implications for neurodegenerative disease research.
The Role of Hevin and Astrocytes in the Brain
Hevin is a protein naturally produced by astrocytes, specialized support cells within the central nervous system. Unlike neurons, which are responsible for transmitting electrical signals, astrocytes maintain and nurture the synapses — the vital connections that enable communication between neurons. These connections are essential for processes like learning and memory, both of which deteriorate in Alzheimer's disease and other forms of dementia.
Shedding light on the importance of astrocytes, neurobiologist Flávia Alcantara Gomes of UFRJ explains, “Hevin is a well-known molecule involved in neural plasticity.” Neural plasticity refers to the brain's ability to reorganize itself by forming new neural connections, a process that underpins memory formation and learning.
Experimental Insights: Boosting Hevin in Mice
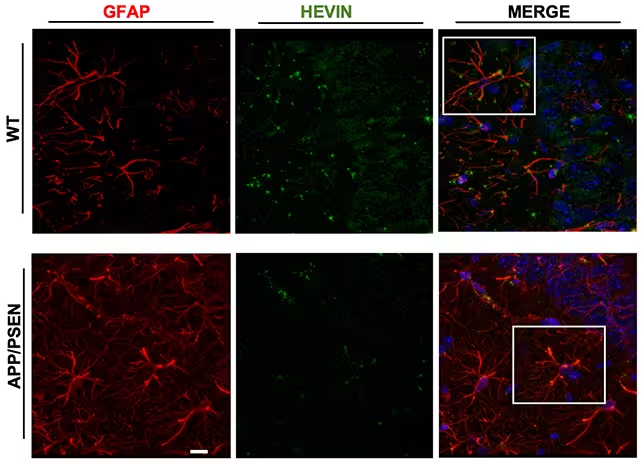
In the controlled study, researchers increased hevin expression in the brains of both healthy mice and mice engineered to develop Alzheimer’s-like symptoms. Over a six-month testing period, these hevin-enhanced animals exhibited substantially improved memory and learning abilities compared to untreated counterparts. Brain imaging further revealed that the increase in hevin led to superior communication across synapses — a marker for efficient neural function.
Analysis of the treated mouse brains suggested that elevated hevin levels triggered the production of additional proteins critical for maintaining healthy synapses. This indicates a ripple effect, where hevin acts in concert with other molecules to support neuronal connectivity and resist cognitive decline.
Broader Impact: Rethinking the Mechanisms of Alzheimer's Disease
To understand the wider relevance of these findings, the research team reviewed public brain data from Alzheimer's patients. They discovered that these individuals tended to have lower hevin levels than people without the disease, reinforcing the hypothesis that astrocyte health and hevin production are integral to cognitive resilience.
Gomes notes, “We’ve shifted the focus from neurons to the critical role of astrocytes, opening new possibilities for treatment strategies targeting these cells in Alzheimer’s and other cognitive disorders.”
Challenging Conventional Theories: Beta-Amyloid Plaques vs. Synaptic Health
Most current Alzheimer’s therapies aim to remove or reduce toxic beta-amyloid plaques — protein clumps that accumulate in the brains of afflicted individuals. However, the new study found that boosting hevin had no effect on plaque formation. This observation aligns with emerging scientific perspectives that beta-amyloid buildup may not be the direct cause of Alzheimer’s disease.
Felipe Cabral-Miranda, a biomedical scientist at UFRJ, comments, “The results support the hypothesis that, while plaques are involved in the disease pathway, they aren’t sufficient to cause Alzheimer’s on their own.” Instead, synaptic failure and cell-to-cell communication breakdown may play a more pivotal role in driving symptoms.
Future Directions and Scientific Significance
While translating these promising laboratory results into safe, effective treatments for humans is a complex and lengthy process, the discovery of hevin’s protective capabilities marks a critical advance. Potential drugs that mimic hevin’s function could eventually be developed to complement or improve upon current dementia therapies.
For now, the key achievement of this research is a deeper understanding of Alzheimer’s cellular and molecular underpinnings. This foundational knowledge could catalyze the development of more holistic, multi-targeted approaches to treating age-related brain disorders.
Conclusion
The identification of hevin as a molecule that can restore cognitive function and protect against synaptic decline in mice signals a promising new frontier in neurodegenerative disease research. By shifting scientific attention beyond neurons to the pivotal role of astrocytes and synaptic health, this discovery expands our toolkit for addressing Alzheimer’s disease and dementia. Although clinical application is still on the horizon, these insights may one day translate into effective therapies that enhance quality of life for millions affected by cognitive impairment worldwide.
The research has been published in Aging Cell.

.avif)
Comments