4 Minutes
Background: New Frontiers in Obesity Treatment
In the evolving field of medical weight loss solutions, medications targeting metabolic pathways have captured global scientific attention. Established drugs such as semaglutide (marketed as Ozempic) have led the way, offering significant weight reduction for individuals with obesity and related metabolic conditions. However, these treatments typically require weekly injections, presenting adherence challenges for many patients.
A new contender, MariTide—or by its scientific name, maridebart cafraglutide—has emerged from recent clinical research as a potential game-changer, requiring just a once-monthly injection.
Clinical Trial Insights: Efficacy of MariTide
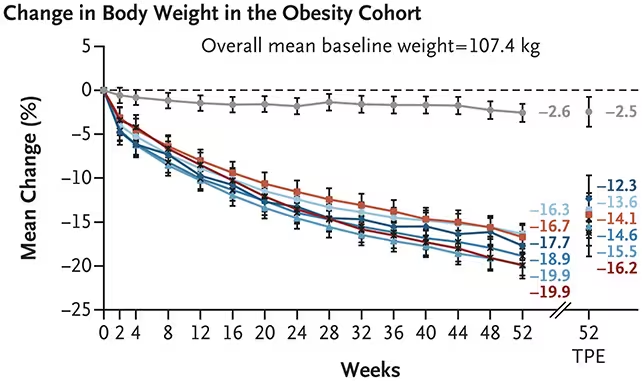
A recent phase 2 clinical trial assessed the safety and effectiveness of MariTide among 465 adults classified as obese. Over a 52-week period, participants receiving monthly doses of MariTide achieved an average weight reduction ranging from 12.3% to 16.2% of their baseline body weight. By contrast, individuals receiving a placebo experienced an average weight loss of only 2.5%.
An additional cohort of 127 participants dealing with both obesity and type 2 diabetes demonstrated similarly encouraging outcomes. Patients in this segment lost between 8.4% and 12.3% of their initial body weight, while the placebo group saw just 1.7% average weight loss over the year.
Lead researchers at the biopharmaceutical company Amgen, collaborating with institutions across the United States and Australia, emphasized the advantages of the once-monthly regimen. Frequent injections have been a barrier to long-term adherence; thus, a monthly schedule could promote sustained engagement and improved patient compliance.
Mechanisms of Action: Targeting GLP-1 and GIP Receptors
MariTide functions in a similar pharmacological class as other weight loss drugs by acting as a glucagon-like peptide-1 (GLP-1) receptor agonist. Activation of GLP-1 receptors in the brain and pancreas is known to suppress appetite and improve blood glucose regulation, both critical factors in managing obesity and metabolic disease.
Remarkably, MariTide also interacts with glucose-dependent insulinotropic polypeptide (GIP) receptors—a relatively novel target in this field. GIP receptors are involved in insulin secretion, fat storage, metabolic rate, and appetite control. This dual-receptor approach may underpin the drug's sustained efficacy and explain its less frequent dosing schedule.
Jay Bradner, executive vice president for research and development at Amgen, highlights the significance: "MariTide delivered strong efficacy, including sustained weight loss without a plateau in the 52-week phase 2 study and meaningful improvements in cardiometabolic risk factors, representing a defining advance for the obesity field."
Safety Profile and Next Steps in Research
While the trial results are promising, side effects were notable. Virtually all participants receiving MariTide encountered at least one adverse effect, predominantly gastrointestinal issues such as vomiting and diarrhea. Notably, these side effects diminished in severity when the dosage was gradually increased to the target level—a strategy that could ease initiation for future patients.
The scientific team underscores that these findings are preliminary and that the journey toward regulatory approval continues. A larger and longer phase 3 trial is expected to further investigate safety, long-term efficacy, and optimal administration strategies.
Potential Impact and Future Directions
Obesity rates are climbing worldwide, bringing significantly increased risk of diabetes, cardiovascular disease, and other health complications. The fact that participants did not reach a weight-loss plateau after one year with MariTide suggests the possibility of even greater long-term benefits. According to the published trial report, "A weight plateau was not reached at 52 weeks, with weight continuing a downward trajectory. Therefore, longer-term trials are needed to assess the full weight efficacy of this agent."
If future results remain positive, MariTide could offer a transformative alternative in the arsenal of anti-obesity therapies, featuring less frequent dosing and broadening access for people in need of sustainable solutions.
Conclusion
MariTide represents a major innovation in the science of weight management drugs, combining robust efficacy, a convenient once-monthly injection schedule, and a dual-mechanism targeting both GLP-1 and GIP receptors. While questions about long-term safety and effectiveness will require further clinical trials, initial results mark a significant advance for obesity treatment. As the global obesity crisis intensifies, advancements like MariTide offer renewed hope for improved metabolic health and quality of life.
Source: doi



Comments