6 Minutes
Background: The Mysterious Nature of Water Ice in Space
Water, a fundamental ingredient for life and a key player in many cosmic processes, continues to surprise scientists with its unusual behaviors. On Earth, we know ice in the familiar crystalline form, with its neatly ordered molecular lattice, easily seen in the intricate symmetry of snowflakes. However, astronomers have long believed that the conditions of deep space—the frigid temperatures and absence of atmospheric pressure—force water to freeze in a far less organized structure known as amorphous ice. This "disordered" ice forms as water vapor directly deposits onto cold surfaces, skipping any liquid phase.
Yet, water ice is far from simple. Researchers have identified at least 20 distinct phases it can adopt depending on pressure and temperature, making it one of the most versatile and enigmatic substances in the cosmos. Understanding exactly how water freezes in space is not merely academic—it holds critical implications for planetary formation, the movement of matter across galaxies, and the detection of habitable environments on other worlds.
Research Methods: Simulating and Experimenting with Extraterrestrial Ice
To probe the microscopic structure of water ice beyond Earth, a team led by physicists at University College London and the University of Cambridge employed a blend of high-powered computer simulations and laboratory experiments. Their goal: to determine whether deep-space ice is truly as featureless as previously believed, or whether hidden order lurks beneath its surface.
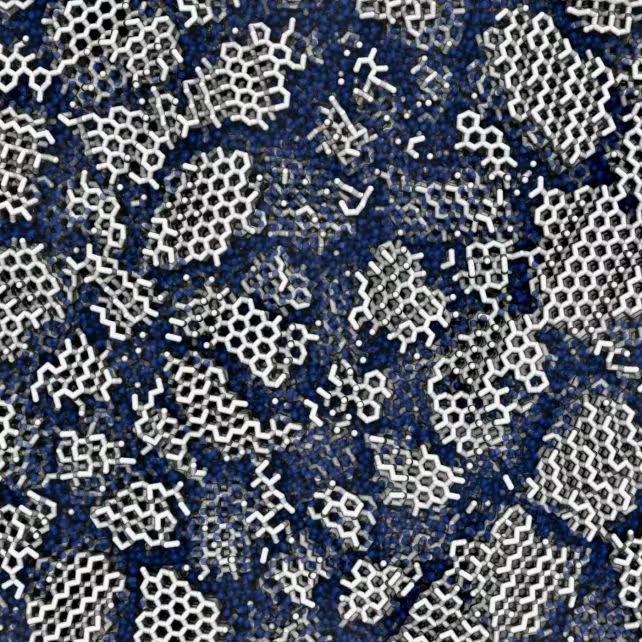
Using molecular dynamics simulations, the researchers virtually "froze" containers of water molecules to temperatures around -120°C (-184°F). By varying cooling rates, they created different blends of amorphous and crystalline ice. Slower cooling allowed more time for molecules to organize into lattices, while rapid freezing resulted in more disorder. Previous work had used X-ray scattering to probe the interior of amorphous ice samples, but these new experiments matched observed structures best when the ice contained about 20% crystalline and 80% amorphous regions—a mix suggesting nanoscale order is present even in supposedly disordered ice.
To mimic cosmic ice formation processes in the lab, the team also deposited water vapor onto ultra-cold surfaces (as would happen on interstellar dust grains or planetary rocks), as well as creating denser forms of amorphous ice by compressing and cooling samples. An important step was gently warming their samples to watch for the emergence of crystalline patterns, an indicator that some internal structure had previously formed, even if it wasn't initially apparent.
Key Discoveries: Crystalline Islands Hidden in Space Ice
Contrary to long-held assumptions, the researchers discovered that ice formed in space is not entirely amorphous. Instead, it contains small, nanometer-scale crystalline domains embedded within its amorphous matrix. These tiny crystals would have formed despite the low temperatures, which were thought insufficient to let molecules arrange themselves into orderly patterns. This surprising structural feature suggests that even in the harshest environments, water finds a way to achieve pockets of order, challenging textbook notions of cosmic ice.
"We now have a good idea of what the most prevalent form of ice in the Universe looks like on an atomic scale," said Dr. Michael Benedict Davies. "This matters because ice plays a vital role in how planets emerge, how galaxies develop, and how material cycles through the Universe."
Crucially, these insights extend beyond astrophysics. Since water "remembers" its previous crystalline arrangements—retaining structural hints even as temperature and phase change—amorphous materials in technology may also conceal hidden crystals. This is highly relevant in fields like fiber optics, where amorphous glass is used to transmit data; the presence of crystals could impact performance.
Broader Implications and Future Perspectives
This research does more than rewrite our picture of interstellar ice. It also opens new questions about the behavior of amorphous materials in general—a class that spans not just ices, but glasses, polymers, and technical ceramics, with countless applications in modern technology. As Dr. Christoph Salzmann of University College London noted, "Ice on Earth is notable for its ordered forms due to our relatively warm planet. Elsewhere in the Universe, ice was assumed to be wholly disordered, a snapshot of liquid water frozen in time. Our work shows that's not exactly the case."
If amorphous materials—from cosmic ices to glass fibers—harbor nano-crystals, then future research could yield methods both to detect and control this structure, potentially improving the performance of devices that rely on disordered solids. The study, while based on Earth-bound experiments, strongly suggests that nanoscale crystalline domains are a common feature of ice throughout the Universe, impacting everything from astrophysical chemistry to technological material science.
Conclusion
The revelation that space ice is not purely amorphous but contains intricate nanocrystals fundamentally alters our understanding of water's behavior in the cold depths of the cosmos. This nuanced picture not only enhances our knowledge of planetary and galactic evolution but also hints at unseen possibilities for the design and optimization of amorphous materials used in advanced technology. As scientists explore further, these findings may help unravel the complexities of both the Universe’s most abundant ice and the technical lifelines of our own digital world.



Comments