5 Minutes
Mitochondria’s evolutionary role as the cell’s energy supplier is well established in biology textbooks. Yet, recent research is shifting this narrative, revealing that mitochondria serve as much more than biochemical generators. New discoveries show mitochondria are pivotal players in the immune system, directly influencing the body’s ability to detect and neutralize bacterial threats.
The Expanding Role of Mitochondria in Cellular Immunity
For decades, mitochondria have primarily been recognized for their role in producing ATP, the cell’s main energy currency. However, modern cell biology demonstrates these organelles also orchestrate complex immune defense mechanisms. Mitochondria participate in inflammation regulation, apoptosis (programmed cell death), and acute responses to microbial invasion, highlighting their crucial contribution to innate immunity.
Recent investigations by immunologists have unveiled a novel function: mitochondria act as sensors within white blood cells, especially neutrophils, detecting bacterial metabolites and activating antimicrobial defenses accordingly. Understanding this newly identified link between cellular metabolism and immune response is advancing the field of immunometabolism—a discipline examining how metabolic processes govern immune functions at the molecular level.
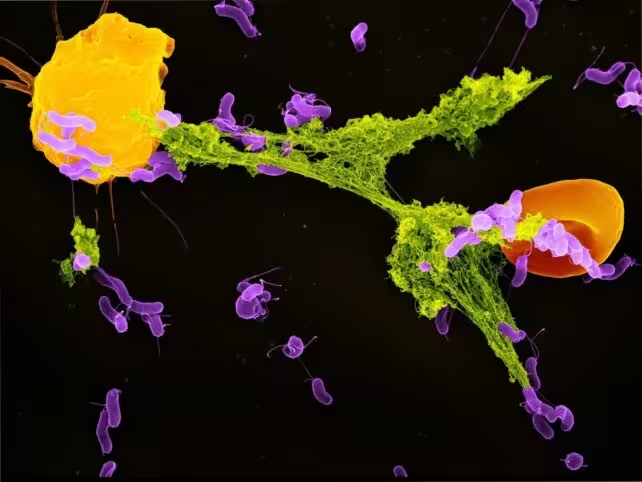
How Neutrophils and Mitochondria Cooperate to Combat Bacteria
Neutrophils, the most abundant type of white blood cell in humans, form the front line against bacterial invaders. One of their unique strategies involves ejecting structures called neutrophil extracellular traps (NETs). NETs consist of webs of DNA coated with antimicrobial proteins, designed to physically ensnare and immobilize invading microbes before they can spread throughout the body.
While the formation of NETs was once attributed mainly to general cellular stress or injury, emerging research reveals a more refined trigger. Scientists have demonstrated that mitochondria within neutrophils are capable of sensing lactate—a metabolic byproduct produced by many bacteria during infection. In humans, lactate is usually associated with muscle fatigue, but in the context of immune response, its presence signals active bacterial metabolism.
When bacteria are engulfed by neutrophils inside special compartments known as phagosomes, the lactate they release becomes detectable to mitochondrial sensors. This metabolic signal prompts mitochondria to instruct neutrophils to deploy NETs, effectively trapping the bacteria. If this mitochondrial sensing mechanism is inhibited, NET production decreases, allowing bacteria to evade capture and proliferate—a critical failure in the immune defense line. This process exemplifies a sophisticated dialogue between bacterial activity and host cell bioenergetics, emphasizing the complexity of microbial-host interactions.
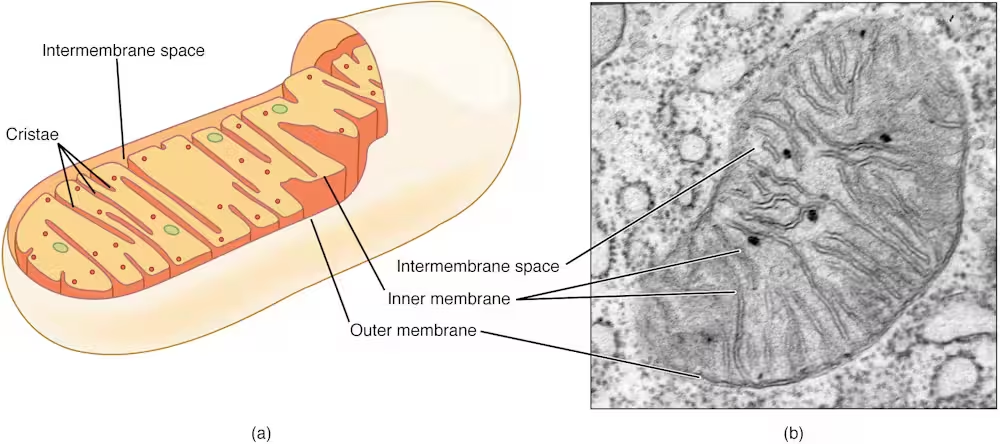
The Scientific Implications: Mitochondrial Dysfunction and Disease Susceptibility
These insights are particularly significant for understanding why certain individuals, such as those with autoimmune disorders like systemic lupus erythematosus (SLE), are more susceptible to recurrent and severe infections. Research has indicated that neutrophils in lupus sufferers often have dysfunctional mitochondria, impairing their ability to sense bacterial lactate and form NETs efficiently. Despite having an immune system in a highly activated state, these patients show reduced antimicrobial vigilance—a paradox explained by mitochondrial malfunction.
This discovery bridges two major issues in immunology: immune system overactivity (as in autoimmune diseases) and immune system weakness (such as increased infection risk). Healthy mitochondria coordinate well-targeted responses, while defective mitochondria disrupt the balance, either tipping the scale toward excessive inflammation or rendering the body vulnerable to pathogens.
Therapeutic Prospects: Targeting Mitochondrial Pathways in Infection Control
The realization that mitochondria are requisite sensors for metabolic signs of infection opens promising avenues for new treatments. Pharmacological agents designed to enhance mitochondrial sensing capability could amplify NET responses, bolstering defense mechanisms in individuals with weakened immunity. Conversely, in diseases where excessive NET formation is detrimental—such as advanced COVID-19 or certain forms of autoimmunity—therapies aiming to dampen this pathway might prevent tissue damage caused by runaway immune reactions.
Furthermore, this research prompts vital questions for the future. Do other immune cell types utilize similar mechanisms to detect pathogen-derived molecules? What other microbial metabolites might play a role in activating or inhibiting immune responses? A deeper understanding of these cellular signaling networks could facilitate the development of strategies that fine-tune immunity—minimizing side effects while maximizing protection against infections.
As researchers continue to unravel the multifaceted roles of mitochondria, it becomes clear that these organelles are not merely passive energy suppliers. Instead, they function as cellular sentinels—watchtowers that detect the slightest biochemical clues from invading bacteria, alerting and coordinating the immune system’s myriad tools to protect the host.
Conclusion
In summary, mitochondria have emerged as vital players in the intricate interplay between cellular metabolism and immune defense. By acting as both energy factories and immune sentinels, these organelles direct how neutrophils and other immune cells detect and respond to bacterial challenges. This radical reimagining of mitochondrial function not only enhances our understanding of human biology at a fundamental level but also unlocks new opportunities for developing precision therapies in infection control and autoimmune disorders. The ongoing exploration of immunometabolism promises to illuminate even more about the dynamic partnerships that safeguard our health at the microscopic level.
Source: theconversation


Comments