4 Minutes
A groundbreaking study from Yale University has uncovered that COVID-19 infection may provoke the accumulation of amyloid beta proteins—a hallmark of Alzheimer’s disease—not only in the brain but also in the eyes. This discovery sheds new light on the biological underpinnings of “brain fog,” a common cognitive symptom experienced by many following a COVID-19 infection, and may have important implications for future Alzheimer’s disease research and diagnosis.
Scientific Background: Brain Fog and Protein Plaques
Many people recovering from COVID-19 report persistent neurological issues such as forgetfulness, confusion, and trouble concentrating, often grouped under the term "brain fog." Researchers have increasingly sought to clarify the mechanisms behind these post-viral cognitive symptoms. Previous Alzheimer’s research has shown that beta-amyloid plaques—sticky protein clumps—build up in the brains and retinas of those with the disease, disrupting neural communication and function. However, until now, there was limited evidence linking viral infections to such plaque formation outside of neurodegenerative conditions.
The Yale Study: Methods and Findings
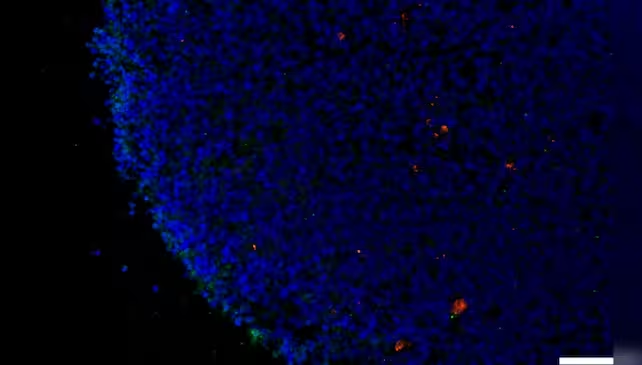
To investigate a possible connection, the Yale-led research team analyzed human retinal tissue collected post-mortem, as well as laboratory-grown retinal organoids (three-dimensional tissue cultures generated from stem cells). Given that the retina is an accessible extension of the central nervous system, it offers a unique opportunity to study molecular changes that mirror those in the brain.
The study focused on two key proteins, neuropilin-1 (NRP1) and angiotensin-converting enzyme 2 (ACE2), which are critical for allowing SARS-CoV-2—the virus causing COVID-19—to enter cells. Utilizing RNA analysis, the researchers identified significant expression of NRP1 in neurons and glial (support) cells of retinal samples from individuals who had contracted COVID-19. This suggests that the virus may exploit NRP1 as a gateway into the cells of both the retina and potentially the brain.
Remarkably, both in retinal tissues from COVID-19 patients without prior dementia and in exposed retinal organoids, researchers found heightened levels of amyloid beta. When retinal organoids were exposed to the virus’s spike protein—the molecule responsible for cell entry—the accumulation of amyloid beta increased significantly. In a promising experimental twist, introducing an NRP1 inhibitor effectively prevented the spike protein from triggering this harmful protein build-up.
Key Implications: Eye and Brain Health, New Therapeutic Targets
The appearance of Alzheimer’s-like plaques in the eyes and brains of COVID-19 patients could revolutionize the way clinicians understand and monitor both diseases. If retinal amyloid beta levels reliably mirror those in the brain, eye exams could emerge as practical, non-invasive tools for early detection of neurodegenerative conditions exacerbated or initiated by viral infections.
More importantly, the study hints at new therapeutic possibilities: targeting NRP1 may help limit or even prevent the neurological complications of COVID-19, including brain fog and potentially long-term cognitive decline. As Dr. Brian Hafler of Yale School of Medicine notes, “The involvement of NRP1 in amyloid beta aggregation provides a specific molecular target for future investigation.”
Amyloid Beta: More Than Just a Disease Marker?
Traditionally blamed for causing Alzheimer’s, amyloid beta is now being reevaluated. Structurally similar to antimicrobial peptides, some scientists theorize that amyloid beta may actually serve a defensive function in the brain, forming in response to infection to trap invading pathogens. This "antimicrobial protection hypothesis" could explain why viral infections, including COVID-19, might stimulate beta-amyloid production.
Given that Alzheimer’s disease is linked to a weakening of the blood-brain barrier, an excess of amyloid beta in the aftermath of a viral infection could represent the brain’s attempt to shield itself from further harm.
Future Prospects: Expanding Research and Clinical Trials
The Yale team is now advancing to clinical studies, aiming to determine whether COVID-19 survivors are at higher risk for long-term neurodegenerative conditions such as Alzheimer’s. Their ultimate goal, according to Dr. Hafler, is to “prevent chronic neurological damage from COVID-19, and to pursue NRP1 inhibitors and similar therapies to guard against virus-induced amyloid pathology and cognitive decline.”
The findings also raise new questions about other viruses' capacity to initiate similar pathological processes. As the medical community continues to assess the lasting impacts of the pandemic, research into the intersection of viral infections, protein aggregation, and neurological health will likely remain a top priority.
Conclusion
This new evidence draws a direct link between COVID-19 infection and the development of Alzheimer’s-like amyloid beta plaques in both the retina and brain, offering new explanations for persistent cognitive symptoms—such as brain fog—seen after the virus. The results not only highlight the retina as a window into brain health but also open doors to new diagnostic tools and targeted therapies aimed at minimizing the neurological aftermath of viral infections. Continued research will be vital to fully understand these mechanisms and to develop effective interventions that protect the brain long after the acute phase of disease has passed.
Source: science


Comments