4 Minutes
Unveiling a New Sensory Pathway: How Gut Microbes Influence the Brain
Recent scientific research is fundamentally reshaping our understanding of the gut-brain connection, revealing a newly classified "neurobiotic sense"—a distinct mode of sensory perception that enables gut bacteria to directly influence brain function, appetite, and behavior. This breakthrough comes from a team of neuroscientists at Duke University, who have identified precise biological mechanisms that facilitate real-time communication between the microbiome in the digestive tract and the nervous system.
Scientific Background: The Gut-Brain Axis and Its Impact
The gut-brain axis, the complex two-way communication system linking the gastrointestinal tract to the central nervous system, has long been implicated in a range of health issues, from metabolic disorders to mental health conditions. However, until now, the underlying physical processes that translate microbial activity into neural signals remained poorly understood. The human digestive system, home to trillions of microorganisms forming the gut microbiome, emerges as a dynamic platform impacting everything from immunity to emotional well-being.
New Experiments Trace the Pathways: Flagellin as a Key Messenger
In their groundbreaking study, Duke University researchers focused on a bacterial protein called flagellin. Flagellin is naturally present in many gut bacteria and is recognized by the immune system as a molecular signal. The study's ambition: to determine whether flagellin could also serve as a direct neural communicator between gut microbes and the brain beyond its known immune role.
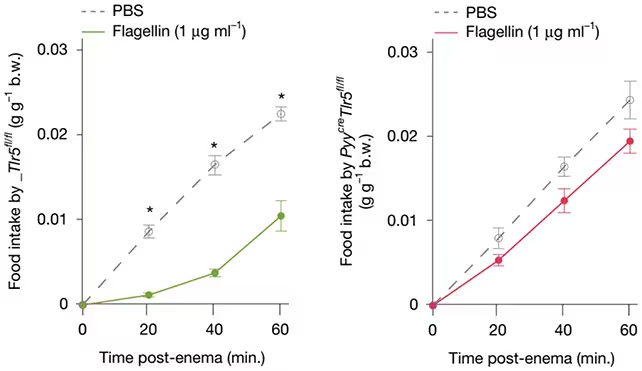
To investigate this, scientists provided mice with small, controlled doses of flagellin following a period of fasting. Observations revealed that these doses triggered a cascade of physiological responses. Specifically, flagellin appeared to engage specialized colon cells called "neuropods," which then transmitted information via the vagus nerve—the main neural conduit connecting the gut to the brain. The subsequent result was a noticeable reduction in food intake, indicating that microbial signals modulate feeding behavior in real time.
Further experiments involved disabling the receptors sensitive to flagellin in the mice. When these receptors were inactivated, the typical reduction in eating behavior did not occur, offering compelling evidence that the flagellin-neuropod-vagus nerve pathway constitutes a vital gut-brain communication channel.
The Proposal of a "Neurobiotic Sense"
Building on these insights, the research team proposes the recognition of a sixth sensory system—the "neurobiotic sense." Unlike the classical senses (e.g., sight, sound, smell), this novel sense allows the body to detect and respond to molecular patterns generated by resident gut microbes. As described in the published study, this sensory system enables an organism to continuously adjust behaviors—such as appetite and possibly even mood—based on microbial cues.
"This sense enables the host to adjust its behavior in response to a molecular pattern from its resident microorganisms," the study authors note. "We call this sense at the interface of the biota and the brain the neurobiotic sense."
Implications for Human Health and Future Directions
Although these initial findings come from rodent models, the structural and functional similarities between rodent and human digestive systems suggest that this neurobiotic signaling is likely to operate in people as well. If further research confirms this, it could transform the way scientists approach the treatment and understanding of disorders linked to the gut-brain axis, such as obesity, eating disorders, depression, and anxiety.
Lead scientist Diego Bohórquez highlights the broader importance: "Looking ahead, I think this work will be especially helpful for the broader scientific community to explain how our behavior is influenced by microbes. One clear next step is to investigate how specific diets change the microbial landscape in the gut. That could be a key piece of the puzzle in conditions like obesity or psychiatric disorders."
Future studies aim to map additional microbial communication pathways impacting the nervous system and to track how shifts in the gut microbiome—potentially driven by diet, age, or lifestyle—alter neurobiotic signaling. As researchers continue to unravel these intricate networks, new strategies may emerge for modulating appetite, metabolism, and mental health by harnessing or modifying this remarkable sixth sense.
Conclusion
This pioneering exploration of the "neurobiotic sense" marks a significant advance in our understanding of gut-brain interplay. By shedding light on the direct, real-time communication between gut bacteria and the nervous system, this research opens up exciting new possibilities for diagnosing, preventing, and treating a wide array of health conditions. As the field expands, targeted interventions at the level of the microbiome may soon offer innovative solutions for optimal brain and body health.
Source: nature


Comments