4 Minutes
Rewinding Cosmic History: Reconstructing the Universe’s First Chemistry
For the first time, scientists have successfully reconstructed the chemical reactions that forged the earliest molecules of the Universe, immediately following the Big Bang. An international team led by physicists at the Max Planck Institute for Nuclear Physics (MPIK) in Germany meticulously recreated these primordial processes using specialized laboratory environments, offering new insights into the foundational chemistry that eventually made stars—and life—possible.
Scientific Background: From Big Bang to Molecule Formation
Roughly 13.8 billion years ago, the freshly born Universe existed as an intensely hot, dense plasma, bustling with fundamental particles but lacking stable atoms. It took approximately 380,000 years for this inferno to cool enough for protons and electrons to merge into the first atomic nuclei, forming simple elements. During this transformative period, hydrogen became the Universe’s dominant building block, closely followed by helium and trace amounts of lithium—elements that remain fundamental to cosmic evolution today.
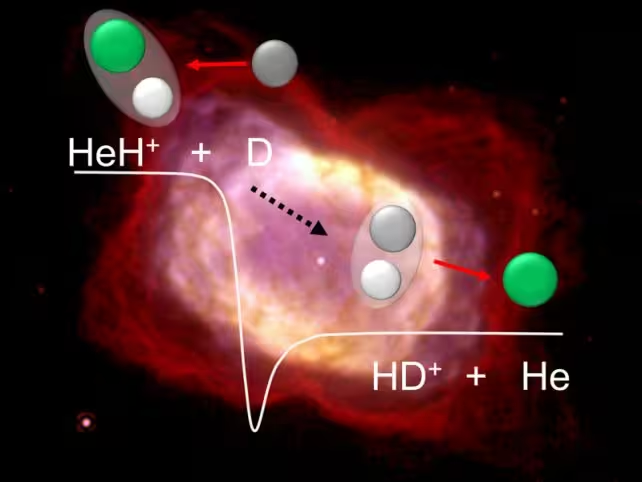
Yet, it was the emergence of the helium hydride ion (HeH+), a molecule comprising a neutral helium atom bonded with an ionized hydrogen atom, that signaled a crucial turning point. This molecule is believed to have played an essential role in the cooling and evolutionary processes that led to the birth of molecular hydrogen (H2)—the most abundant molecule in the cosmos and the raw material for star formation.
The Role of Helium Hydride Ion (HeH+) in Early Star Formation
HeH+ is of special interest to astronomers and astrochemists due to its unique structure, featuring a pronounced separation of electric charges. This separation made it exceptionally efficient at radiating energy away in the presence of cosmic electric fields, thus cooling the primordial gas and paving the way for molecular clouds to condense into star-forming regions under gravity.
Without the presence of HeH+, it is unlikely that the early Universe would have cooled efficiently enough to form dense molecular seeds, delaying the entire process of star and galaxy formation.
Replicating the Baby Universe: The Cryogenic Storage Ring Experiment
To mimic the conditions of the nascent Universe, the MPIK team used a state-of-the-art Cryogenic Storage Ring. This advanced facility allows experiments at ultra-low temperatures—just a few degrees above absolute zero (about -267°C or -449°F)—within a near-perfect vacuum, closely resembling the vast emptiness of interstellar space.
In these conditions, the researchers investigated reactions involving helium hydride ions and deuterium, a heavy isotope of hydrogen with an extra neutron. By precisely controlling particle beams of HeH+ and neutral deuterium, they observed the interactions that yield neutral helium atoms and ionized hydrogen-deuterium molecules (HD+), key intermediates with significant evolutionary implications for the early Universe.
To further their understanding, the scientists adjusted the collision energies—equivalent to varying temperatures—to test how effectively these reactions unfolded in the coldness of space.
Key Discoveries and Astrophysical Implications
Contrary to previous theoretical predictions, the study found that the efficiency of the HeH+ reaction remained unchanged even as temperatures dropped—a finding with profound implications. This suggests that HeH+ was consistently influential throughout the cooling phase, maintaining its role in catalyzing the chemical conditions necessary for the formation of molecular hydrogen and, ultimately, the first stars.
Physicist Holger Kreckel of the Max Planck Institute commented, "Earlier models anticipated a sharp reduction in reaction rates at lower temperatures, but neither our experiments nor refined calculations support this. It turns out that HeH+ interactions with hydrogen and deuterium likely had a much greater impact on early Universe chemistry than previously recognized."
These groundbreaking experimental results not only validate long-standing theories about primordial molecules but also challenge models concerning the temperature dependence of key cosmic reactions. Understanding these processes refines our broader picture of star formation, cosmic chemistry, and the evolution of galaxies.
Looking Ahead: The Frontier of Laboratory Astrochemistry
The ability to emulate conditions from the Universe’s infancy in laboratory settings represents a significant leap forward for astrophysics and astrochemistry. The outcomes from this research pave the way for deeper explorations into the molecular universe—offering fresh perspectives on how cosmic structures, from the smallest molecules to the largest galaxies, took shape after the Big Bang.
As researchers continue to probe fundamental reactions at extreme conditions, we can expect a more nuanced understanding of the chemical processes underpinning the cosmos, potentially informing the search for complex molecules and even life beyond Earth.
Conclusion
By recreating the Universe's first chemical reactions under laboratory conditions reminiscent of the early cosmos, scientists have shed crucial new light on the origins of molecular hydrogen and star formation. The pivotal role of helium hydride ions (HeH+) is now more apparent than ever, affirming their status as critical agents in cosmic evolution. This work not only answers longstanding questions about early Universe chemistry but also sets the stage for future explorations into the molecular foundations of our cosmic heritage.
Source: aanda



Comments