5 Minutes
a single amino acid and the puzzle of calorie restriction
Cutting caloric intake is a proven route to weight loss, but the precise biological signals that trigger fat to burn calories have remained incompletely understood. A multinational team of researchers based in the United States has identified cysteine — a sulfur-containing amino acid abundant in many protein-rich foods — as a key regulator in this process. Their work, published in Nature Metabolism, suggests that lowering cysteine levels can convert energy-storing white adipose tissue into energy‑dissipating brown adipose tissue, effectively switching on a heat-generating, calorie-burning program in the body.
Experiment design and main findings
To test causality, the team used a mouse model genetically unable to synthesize cysteine and controlled dietary cysteine intake. When dietary cysteine was withheld, these mice lost an extraordinary 25–30% of body weight within a single week compared with control animals that could produce or receive cysteine through their diet. Reintroducing cysteine restored weight and metabolic balance, indicating the weight loss was directly tied to cysteine availability rather than irreversible damage.
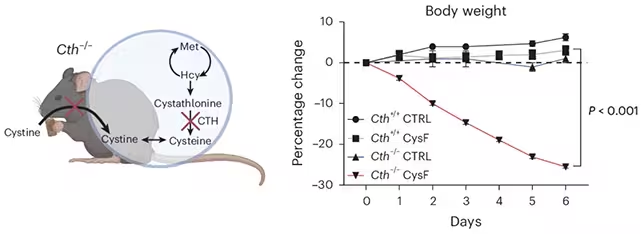
Mice with reduced cysteine lost a lot of weight very quickly. (Lee et al., Nat. Metab., 2025)
To explore human relevance, researchers re‑analyzed tissue samples and metabolic data from 238 participants in an earlier calorie‑restriction trial. Adipose tissue from people undergoing caloric restriction showed lower cysteine levels, supporting the hypothesis that reduced dietary calories lower cysteine and promote fat browning in humans as well.
Mechanisms and metabolic trade‑offs
White-to-brown adipose conversion
White adipose tissue (WAT) stores energy as fat; brown adipose tissue (BAT) contains abundant mitochondria and specialized proteins that dissipate energy as heat — a process called thermogenesis. The new study shows cysteine depletion activates gene programs and mitochondrial changes in WAT that resemble BAT, increasing whole‑body energy expenditure.
Redox biology and safety concerns
Cysteine is not only a substrate for protein synthesis; it is central to cellular redox balance (via glutathione and related pathways). The study authors and independent experts caution that indiscriminate blocking of cysteine could destabilize oxidative balance and cause severe systemic harm. In mice, extreme cysteine restriction produced dangerously rapid weight loss, although the effect was reversible with cysteine repletion.
"These results suggest future weight management strategies that might not rely exclusively on reducing caloric intake," said Krisztian Stadler of the Pennington Biomedical Research Center. Eric Ravussin added that reverse translation from a human caloric restriction trial identified a new player in energy metabolism.
Implications for therapies and nutrition
Targeting amino‑acid metabolism is a promising but delicate approach. Potential applications include precision nutrition plans that modulate cysteine intake, or small molecules that transiently alter cysteine metabolism in adipose tissue to increase thermogenesis. Any therapeutic strategy must carefully balance benefits against risks to redox homeostasis and other cysteine‑dependent processes.
Related technologies that could accelerate translation include targeted delivery of metabolic modulators to adipose tissue, metabolomics to monitor cysteine flux in patients, and gene‑therapy vectors to control localized enzyme activity in fat depots.
Expert Insight
Dr. Maya Chen, a metabolic physiologist (fictional) commented: "This study elegantly links a specific nutrient signal to a powerful metabolic outcome. It reframes calorie restriction partly as a change in nutrient signaling — not just energy deficit. However, translating this to humans will require precise tools to nudge cysteine pathways in adipose tissue without disrupting systemic antioxidant defenses."
Conclusion
The discovery that cysteine depletion can flip white fat into brown‑like, calorie‑burning tissue identifies a previously hidden metabolic switch that helps explain how caloric restriction reduces body weight. While the findings open new avenues for weight‑management strategies and metabolic therapies, they also underscore substantial safety challenges: cysteine is central to redox biology and broad metabolic health. Future research will need to define safe, tissue‑specific ways to harness this switch for clinical benefit. The full study is available in Nature Metabolism.
Source: sciencealert

Leave a Comment