6 Minutes
a possible universal approach to cancer vaccination
Researchers at the University of Florida report a preclinical advance that could broaden the reach of therapeutic cancer vaccines. Rather than instructing the immune system to recognize a single tumor-specific target, the new mRNA-based vaccine primes a generalized, powerful immune alarm that makes a range of tumor cells — including treatment-resistant types — more visible to immune attack. The study, published in Nature Biomedical Engineering, shows durable tumor control and complete regression in some mouse models when the vaccine is combined with immune checkpoint inhibitors.
Scientific background and mechanism
Cancer vaccines aim to train the immune system to identify and destroy malignant cells. Conventional therapeutic vaccines typically present tumor-specific antigens so T cells can recognize a defined marker. However, many cancers evade detection by varying those markers or by creating an immunosuppressive microenvironment.
The new strategy uses an mRNA formulation (described by the authors as uRNA) that encodes immune-stimulating signaling proteins rather than tumor-specific antigens. When administered, the mRNA instructs cells at the injection site to produce cytokines and other immunomodulators that turn on innate and adaptive immune pathways. The effect is to "wake up" dormant antigen-presenting cells and recruit them into a sustained anti-tumor response.
Crucially, the molecules produced from the vaccine's mRNA are not tailored to a given tumor. Instead, they amplify responses from otherwise quiescent immune clones and boost recognition of a broader array of tumor-associated features. In metaphorical terms, the vaccine acts as a universal siren that alerts the body's border patrol — elevating surveillance across different tumor types and genetic backgrounds.
Immune checkpoint blockade as a partner therapy
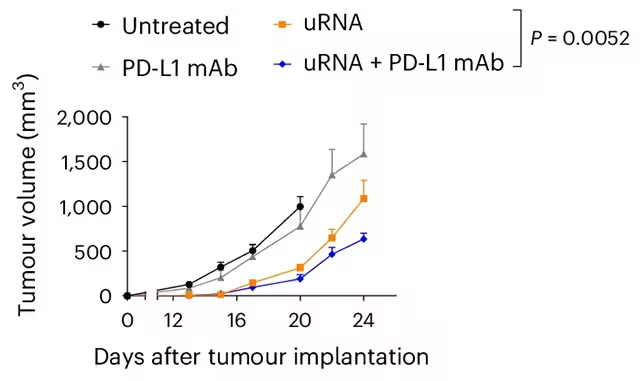
The study paired the mRNA vaccine with immune checkpoint inhibitors (ICIs) — specifically PD-L1–targeting monoclonal antibodies (PD-L1 mAb). Checkpoint inhibitors remove molecular brakes that suppress T cell activation, and when combined with a vaccine-induced increase in immune priming, they can produce a synergistic effect.
Many tumors with high mutational loads or adaptive resistance mechanisms evade ICIs alone. In the mouse experiments, the combination of uRNA and PD-L1 mAb triggered strong anti-tumor immunity, reversing resistance in several aggressive models and eliminating some tumors outright. The authors emphasize that while the vaccine showed activity as a monotherapy in some settings, the most robust and reproducible responses occurred in combination with checkpoint blockade.
Key discoveries, implications and next steps
Key findings from the preclinical work include:
- Broad immune sensitization: The mRNA vaccine mobilized diverse immune cell types and improved detection of tumor cells not previously targeted by existing vaccines.
- Overcoming resistance: Tumors known to resist checkpoint blockade were rendered susceptible when the vaccine preceded or accompanied ICI therapy.
- Complete regressions: A subset of mice achieved full tumor clearance, indicating potential curative effects in particular models.
The implications are significant for cancer immunotherapy. A broadly acting, "off-the-shelf" vaccine could reduce the need to design patient-specific neoantigen vaccines and expand access to immunotherapy for patients whose tumors lack clear targetable antigens. As oncologist Elias Sayour, quoted in the study, notes: this approach provides a proof of concept for commercializable universal cancer vaccines that sensitize the immune system against individual tumors.
However, the findings remain preclinical. Critical next steps include dose optimization, safety profiling, and carefully designed human clinical trials to assess efficacy and immune-related adverse events. The team is developing new mRNA formulations and planning trials to evaluate both treatment and recurrence-prevention scenarios.
Safety, selection and limitations
Modifying immune activation carries well-known risks. Excessive or misdirected immune stimulation can cause autoimmunity, cytokine release syndromes, or organ-specific toxicities. Determining therapeutic windows and patient selection biomarkers will be essential to mitigate these risks. The researchers are also investigating whether specific immune or genetic signatures predict better responses to the vaccine-plus-ICI regimen.
Additional limitations of the current work include the constraints inherent to mouse models, which do not fully recapitulate human tumor heterogeneity, microenvironments, or long-term immune regulation. Translational challenges — manufacturing, stability, and regulatory approval pathways for a universal immunomodulatory mRNA product — will also need addressing.
Expert Insight
Commentary from a science communicator
"This study is an important conceptual shift: instead of designing a vaccine to match the tumor, it amplifies the immune system's ability to notice the tumor in the first place," says Dr. Amina Patel, an immunologist and science communicator. "If replicated safely in humans, such a strategy could simplify how we deploy immunotherapy across many cancer types — but the safety profile and careful patient selection will determine its clinical value."
Future prospects and related technologies
The work sits at the intersection of mRNA therapeutics, tumor immunology, and immune-oncology drug development. It complements other innovations such as personalized neoantigen vaccines, CAR-T cell therapy, and combination regimens that include oncolytic viruses or targeted small molecules. Advances in mRNA delivery platforms, improved adjuvant design, and predictive biomarkers for immune responsiveness will accelerate translation.
Researchers are also exploring whether similar vaccine principles could prevent recurrence after surgery and whether different checkpoint targets or dosage schedules further improve outcomes. Collaboration between academic groups, biotech companies, and clinical consortia will be crucial to move this concept into human trials.
Conclusion
The University of Florida-led preclinical study describes an mRNA vaccine strategy that broadly activates anti-tumor immunity and, when combined with PD-L1 checkpoint blockade, eliminates treatment-resistant tumors in mice. The results support the concept of a universal cancer vaccine that enhances immune recognition across diverse tumors. While promising, the approach requires careful clinical testing to evaluate safety, optimal combinations, and which patients are most likely to benefit. Published in Nature Biomedical Engineering, the study marks an important step toward expanding the reach of cancer immunotherapy and highlights the continuing promise of mRNA technology in oncology.
Source: sciencealert

Leave a Comment