5 Minutes
Genetic evidence reshapes the story of gout
Gout has long been stigmatized as a disease of excess—blamed on alcohol, rich food, or poor lifestyle choices. New genomic evidence, however, shows heredity plays a far larger role than previously recognized. In a 2024 international analysis of genetic data from 2.6 million people spanning 13 cohorts, researchers identified 377 genomic regions (loci) associated with gout; 149 of those loci were novel discoveries. The dataset included 120,295 individuals with prevalent gout. (Boy_Anupong/Moment/Getty Images)
By comparing the DNA of people with and without gout, the team performed a large-scale genome-wide association study (GWAS) that highlighted many genetic variants tied to uric acid regulation and immune responses. These findings move gout toward the domain of precision medicine: understanding which genetic pathways increase risk could steer both prevention and treatment strategies.
Gout arises when elevated levels of uric acid in the blood precipitate into monosodium urate crystals in joints. Those needle-like crystals trigger an inflammatory reaction when the innate immune system recognizes and attacks them, causing acute pain, swelling and long-term joint damage if not controlled. The new genetic evidence implicates genes involved in two key stages: transport and clearance of uric acid, and the immune system’s propensity to respond to urate crystals.
The research team argues that, while diet and environment remain relevant, genetics are major drivers of gout susceptibility and severity. That distinction matters for public health messaging: framing gout primarily as a genetic disease can reduce stigma and encourage patients to seek medical care rather than remaining silent about symptoms.
Implications for treatment, repurposing drugs and public health
The study broadens therapeutic options by identifying molecular targets linked to both urate metabolism and immune activation. Existing urate-lowering drugs—such as allopurinol and febuxostat—address uric acid production or clearance but do not directly modify the immune response to crystals. The GWAS loci tied to immunity suggest that some immunomodulatory agents, already approved for other inflammatory conditions, could be repurposed to reduce attacks or progression in gout.
Clinical and research directions
- Genotype-informed risk assessment: Genetic markers could help identify people at high lifetime risk for gout, prompting earlier monitoring of serum urate and timely preventive therapy.
- Immune-targeted approaches: Drug development or repurposing that modulates pathways implicated by the GWAS may reduce the intensity or frequency of gout flares.
- Population-scale screening: Larger, more diverse genomic studies and functional experiments are needed to translate associations into actionable treatments.
The authors emphasize practical barriers: many patients avoid care because of shame or the misconception that gout is solely self-inflicted. The research team notes that correct framing can increase uptake of preventive therapies that lower serum urate and prevent painful flares.
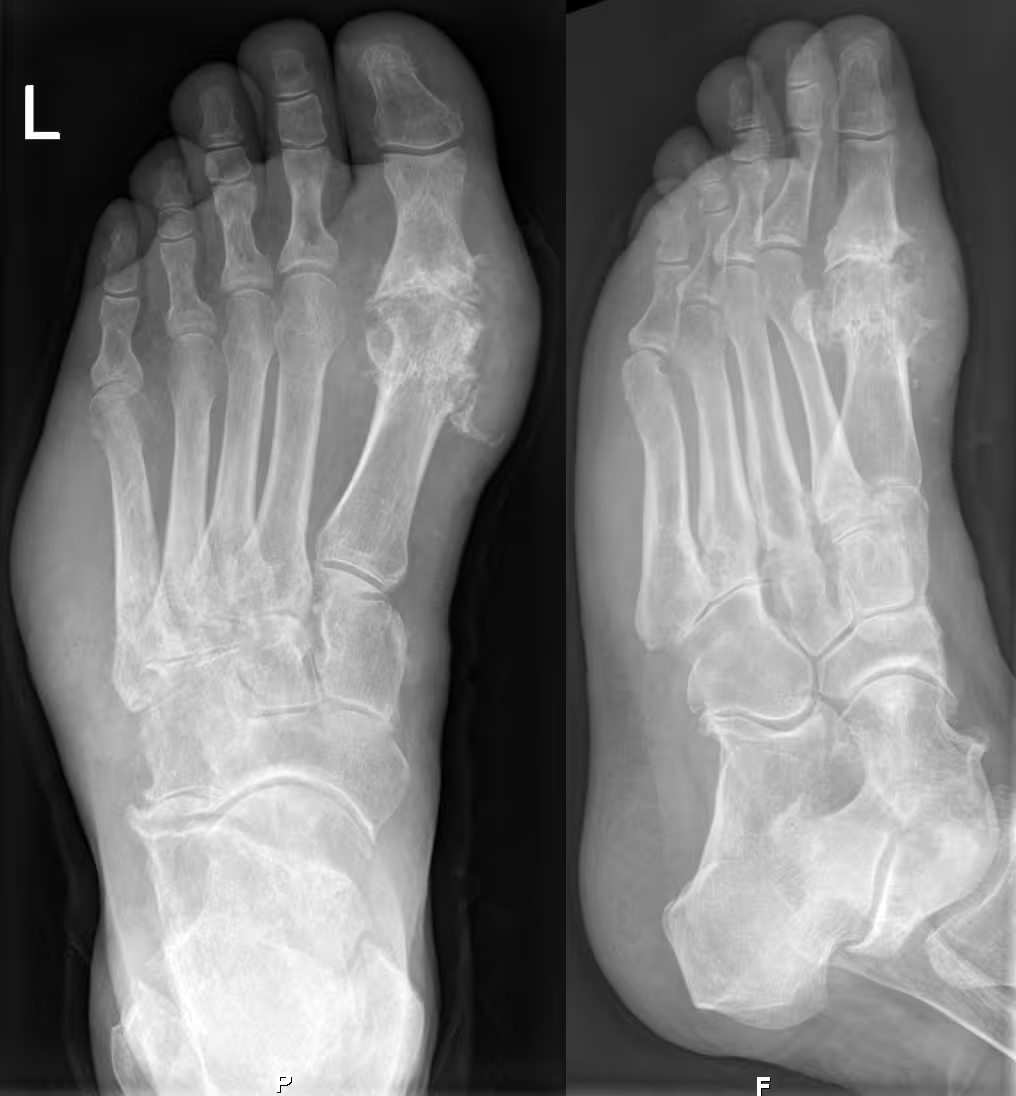
Gout on X-rays of a left foot in the metatarsal-phalangeal joint of the big toe. (Hellerhoff/CC BY-SA 3.0/Wikimedia Commons)
The study does have limitations. Most participants were of European ancestry, which reduces the immediate generalizability of specific loci to other populations. Some gout diagnoses in the cohorts relied on self-report rather than standardized clinical criteria, which may introduce misclassification. Despite these constraints, the analysis provides the most comprehensive genetic map of gout to date and identifies targets for follow-up functional studies and clinical trials. The research was published in Nature Genetics.
Expert Insight
Dr. Laura Martínez, consultant rheumatologist and clinical genomics advisor, comments: "This level of genomic resolution changes how we think about prevention and treatment. We already have effective urate-lowering medications, but knowing the genetic architecture means we can stratify risk and investigate therapies that modify immune pathways driving painful flares. Importantly, communicating that gout has a strong genetic component reduces patient blame and can increase adherence to preventive care."
Dr. Martínez adds that future work must expand datasets to include more diverse ancestries and pair GWAS results with laboratory studies to establish causality and druggability of newly discovered targets.
Conclusion
The largest genomic analysis of gout to date underscores that genetics substantially shapes risk, from uric acid transport to the immune reaction to urate crystals. While lifestyle influences remain relevant, this evidence rebalances clinical and public perceptions of gout away from stigma and toward biology-informed prevention and therapy. Follow-up studies—especially in diverse populations and functional assays—will be essential to convert genetic signals into better diagnostics, repurposed drugs and targeted treatments that can reduce the global burden of this centuries-old disease.
Source: sciencealert

Leave a Comment