5 Minutes
New neural circuit links sleep to body repair
When we sleep, the body shifts into repair mode: growth hormone (GH) is released to rebuild muscle and bone and to regulate metabolic processes. Until recently, scientists measured GH in blood samples taken across sleep, but the neural circuits that trigger and modulate that release remained unclear. A UC Berkeley-led team has now recorded neural activity in mice across multiple sleep–wake cycles and identified a circuit that times growth hormone secretion differently during REM and non-REM sleep.
Scientific background and experimental approach
The team used continuous neural recordings in mice alongside hormonal assays to map how specific neuronal populations correlate with GH pulses. By tracking activity in hypothalamic neurons and nodes that influence arousal, researchers observed distinct patterns of GH regulation in rapid eye movement (REM) sleep versus non-REM sleep. The work was detailed in Cell in 2025 and builds on long-standing evidence that sleep quality directly affects endocrine function and metabolic health.
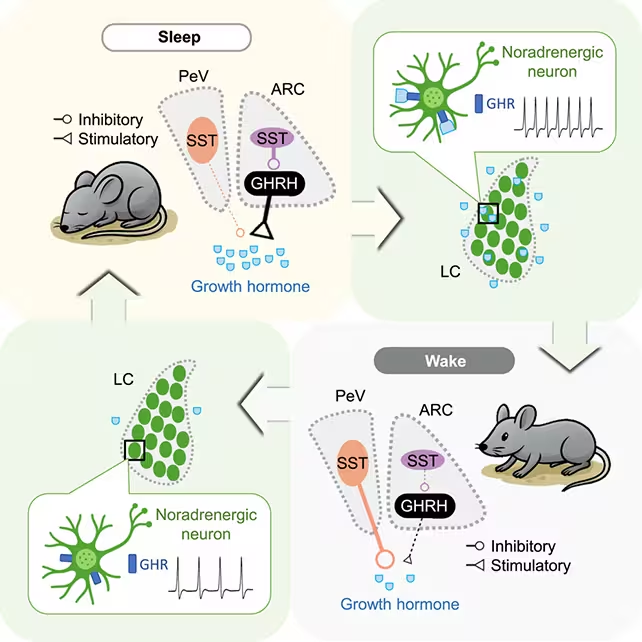
The researchers analyzed the release of growth hormone in mice during sleep/wake cycles.
Key technical points
- Continuous electrophysiological and optogenetic recordings allowed precise timing between neuronal firing and GH pulses.
- The study differentiated neurons that promote GH release from those that inhibit it, showing dynamic shifts in influence across sleep stages.
- Importantly, the locus coeruleus—a brainstem nucleus central to wakefulness—was found to form a feedback loop with GH-regulating circuits.
Main findings and physiological implications
Growth hormone increased during both REM and non-REM sleep, but the neuronal drivers and suppressors of that release changed their relative influence depending on sleep stage. The discovery of a reciprocal loop with the locus coeruleus suggests a tightly balanced system: sleep promotes GH secretion, and GH in turn feeds back to influence arousal. If this balance is disrupted—by chronic sleep loss or circuit dysfunction—metabolic consequences follow.
GH is not only essential for growth and tissue repair; it also modulates glucose and lipid metabolism. Insufficient or mistimed GH release, as can occur with fragmented sleep, is linked to higher risk of obesity, insulin resistance, type 2 diabetes, and cardiovascular disease. The new circuit-level insights therefore connect basic sleep neurobiology to endocrine regulation and long-term health.
Potential clinical and research applications
Understanding the neural circuitry that times GH release opens new therapeutic possibilities. Targeted pharmacology or neuromodulation could restore normal GH rhythms in people with sleep disorders or metabolic disease. The researchers suggest the locus coeruleus may be a promising target: dialing down its excitability could rebalance GH timing and improve sleep continuity.
Translating mouse findings to humans will require further validation, but the conserved role of hypothalamic and brainstem structures in sleep and endocrine control offers a plausible path. Future work may explore whether altered GH–sleep feedback contributes to neurodegenerative conditions such as Alzheimer’s disease, where sleep disruption and impaired metabolic regulation are common.
Expert Insight
"These recordings give us a mechanistic glimpse into how sleep-stage transitions gate hormonal pulses," says Dr. Elena Martinez, a sleep neuroscientist unaffiliated with the study. "If similar circuits exist in humans, there are clear opportunities to refine treatments that target both sleep quality and metabolic health—two interconnected problems in modern societies."
Dr. Martinez adds that emerging tools such as cell-type specific gene modulation and noninvasive brain stimulation could be used to test whether adjusting locus coeruleus activity restores normal growth hormone rhythms without major side effects.
Limitations and next steps
The study establishes a foundation but does not prove identical circuit behavior in humans. Longitudinal human studies combining polysomnography, hormonal sampling, and imaging or neuromodulation will be needed. Researchers also need to determine how aging, obesity, and neurodegeneration modify this sleep–GH loop.
Conclusion
This research maps a neural circuit that times growth hormone release across sleep stages and identifies the locus coeruleus as a feedback node linking endocrine signaling to arousal. By revealing how REM and non-REM sleep differently engage promoters and inhibitors of GH, the study bridges sleep physiology and metabolic health. Continued investigation could lead to targeted therapies for sleep disorders, metabolic disease, and conditions associated with disrupted sleep and hormone balance.
Source: sciencealert


Leave a Comment