8 Minutes
exceptional lifespan and what it can teach us
There is no escape from aging, yet a small number of people — supercentenarians who reach 110 years and beyond — appear to postpone many age-related diseases. A comprehensive molecular and clinical analysis of Maria Branyas, who lived to 117, offers a rare window into the biological features that may support extreme human longevity. The case combines clinical measures, multi-omic profiling and genetic analysis to identify biomarkers linked to healthy aging, immune robustness and cardiovascular resilience.

Maria Branyas in 1925
The research, led by teams at the Josep Carreras Leukaemia Research Institute in Barcelona and published in Cell Reports Medicine, used blood, saliva, urine and stool collected before Branyas' death in 2024. The investigators — including epigeneticists Eloy Santos-Pujol and Aleix Noguera-Castells — report that some of Branyas' cells and molecular signatures "behaved" as if they were much younger than her chronological age. Her profile combines favorable genetics, a low-inflammatory state, a distinctive gut microbiome and cardiovascular markers associated with lower disease risk.
Study design and methods: samples, analyses and comparative frameworks
To explore the biological underpinnings of extreme lifespan, researchers performed a multimodal analysis: whole-genome sequencing, epigenetic age estimates (methylation clocks), lipid profiling, immune-cell phenotyping and gut microbiome sequencing. These assays were compared to reference data from younger cohorts and other elderly individuals to identify deviations linked with resilience rather than decline.
Sample types and laboratory approaches
- Blood: genomic DNA, immune-cell counts, inflammatory markers and lipid panels.
- Saliva: supplementary DNA for sequencing and validation of germline variants.
- Urine: metabolic byproducts and renal function biomarkers.
- Stool: microbiome composition and diversity by 16S rRNA and shotgun metagenomics.

Epigenetic clocks estimate biological age by measuring DNA methylation patterns across the genome; shorter telomeres — the protective caps at chromosome ends — were also profiled to assess cellular aging dynamics. Together, these molecular readouts provide a layered view of aging biology: genetic predisposition, epigenetic regulation, immune health and microbiome interactions.
Key findings: a young molecular profile, favorable lipids and low inflammation
Several consistent signals emerged from the analysis. First, despite her advanced years, many of Branyas' cellular and molecular markers matched those observed in much younger individuals. Her epigenetic age estimates were lower than her chronological age in certain tissues, indicating a slower rate of epigenetic aging in specific cell types. Second, she exhibited exceptionally low systemic inflammation — a known predictor of better health in older adults.
Cardiometabolic markers were also notable: Branyas had very low levels of low-density lipoprotein (LDL) cholesterol and triglycerides, together with unusually high levels of high-density lipoprotein (HDL) cholesterol. This lipid profile aligns with reduced cardiovascular risk and may have contributed to her prolonged healthspan.
Her gut microbiome resembled that of younger cohorts on key metrics, including microbial diversity and the presence of taxa associated with anti-inflammatory metabolites. The researchers also identified rare genetic variants in Branyas' genome linked to longevity, immune function and neuroprotection, suggesting a genetic contribution to her resilience.
Curiously, the team observed a "huge erosion" in Branyas' telomeres. While shorter telomeres are often associated with increased mortality risk, emerging evidence suggests telomere length is not universally predictive among the oldest old. The authors hypothesize that very short telomeres in cell lineages with high proliferative potential may actually limit the chance for malignant clones to expand, offering a potential protective effect against cancer.
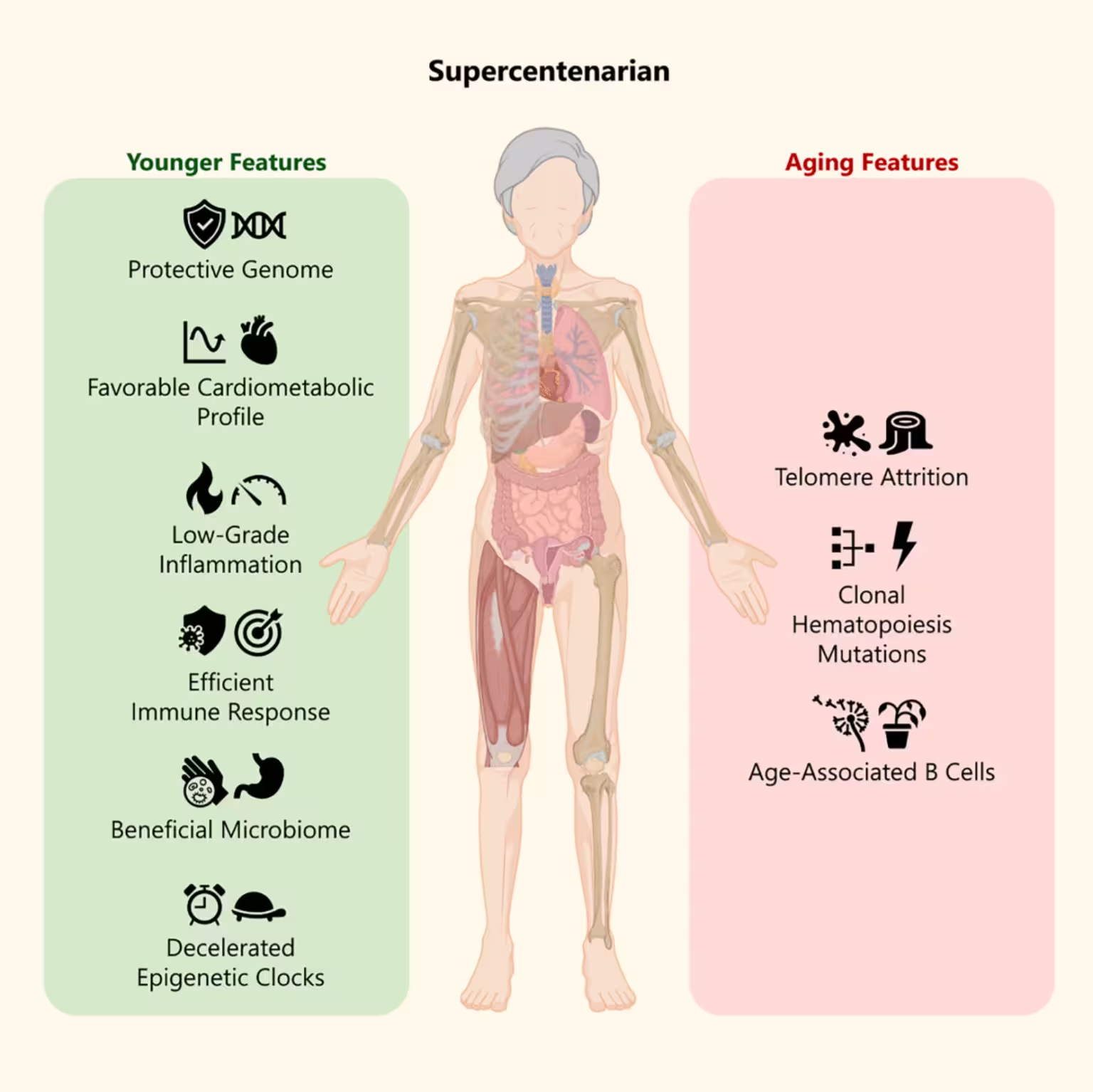
Younger features and aging features of Maria Branyas
Taken together, the most striking picture is one of mixed aging signals: molecular youthfulness in immune and epigenetic clocks, favorable lipid and inflammatory profiles, a microbiome aligned with health, and paradoxically short telomeres. Such a constellation may help explain how Branyas outlived average life expectancy for women in Catalonia by more than three decades.
Scientific context and implications for aging research
This case study contributes to several important themes in longevity science. First, extreme longevity is multifactorial: a combination of protective genetics, lifestyle (for example, a Mediterranean diet higher in fermented dairy like yogurt), low chronic inflammation and resilient immune function. Second, no single biomarker fully captures aging; telomere length, epigenetic age and microbiome composition each provide partial and sometimes conflicting information.
Researchers caution about overgeneralizing from a single individual. Case studies are hypothesis-generating rather than definitive. Larger cohorts of centenarians and supercentenarians are required to identify reproducible biomarkers and causal mechanisms that could translate into interventions to improve healthspan in the general population.
The investigators explicitly suggest that their findings can "provide a fresh look at human aging biology, suggesting biomarkers for healthy aging, and potential strategies to increase life expectancy." Future work will need to validate the identified genetic variants and molecular signatures across independent samples and to test whether modulating the microbiome, inflammation or epigenetic states can measurably extend healthy years in clinical trials.
Expert Insight
Dr. Ana Cortes, a fictional geroscience researcher and science communicator, commented on the study's broader relevance: "Single-case studies like Maria Branyas' are invaluable because they push us to ask why some people defy typical aging trajectories. This work combines genomics, epigenetics and microbiome science in a way that points toward integrated models of resilience. While we cannot generalize from one person, these molecular signatures give us targets to study in larger populations — especially pathways regulating inflammation and immune surveillance."
Her perspective echoes the cautious optimism of the research team: the biology of extreme longevity is complex, but measurable features — from lipid metabolism to microbial metabolites and epigenetic regulation — can guide future translational research.
Practical takeaways and future prospects
For clinicians and researchers, the study highlights candidate biomarkers to prioritize in cohort studies of long-lived people: epigenetic clock measures, inflammatory cytokine profiles, lipid fractions and microbiome diversity metrics. For the general public, the results reinforce well-established recommendations associated with healthy aging: maintain social and cognitive engagement, pursue regular physical activity, follow a balanced diet (the Mediterranean pattern is repeatedly linked to cardiovascular health), and manage chronic inflammation and metabolic risk factors.
On the technological front, this research exemplifies the value of integrated multi-omic approaches — combining genomics, epigenomics, metabolomics and microbiomics — to build a systems-level understanding of aging. Advances in single-cell profiling, longitudinal cohort sampling and interventional trials (for example, microbiome modification or targeted anti-inflammatory therapies) will be essential to move from observational findings to actionable therapies.
Conclusion
The molecular portrait of Maria Branyas shows that extreme age and preserved health can coexist. Her case underlines the complexity of aging: favorable genetic variants, a young-appearing epigenome in some cell types, low inflammation, a resilient microbiome and advantageous lipid levels together paint a picture of biological resilience. While this single case cannot establish causality or generalize to all populations, it provides a valuable roadmap for future studies aimed at identifying biomarkers and interventions that promote healthy aging and extend human healthspan.
The growing population of centenarians worldwide makes these investigations increasingly urgent. With careful replication and expanded cohorts, the molecular clues found in exceptional individuals may translate into strategies that help more people live longer, healthier lives.
Source: sciencealert

.webp)
Leave a Comment