7 Minutes
Rapid mitochondrial 'power-up' helps cancer cells survive mechanical stress
Cancer cells unleash a rapid burst of energy when physically squeezed, triggering mitochondria to rush around the nucleus and deliver extra ATP. This newfound mechanism, observed in both lab experiments and patient biopsies, helps cells repair DNA damage and survive extreme stress.
A study published in Nature Communications describing work at the Centre for Genomic Regulation (CRG) in Barcelona reports that mechanical confinement prompts an immediate increase in nuclear ATP supplied by mitochondria. This fast response appears to protect the genome when cells are mechanically deformed — for example, while squeezing through tight spaces in the tumor microenvironment or during entry into blood vessels — and may be a previously unrecognized contributor to cancer cell survival and invasion.
Experimental approach and key observations
Researchers used a custom live-cell microscopy setup that physically compresses individual cells to roughly three microns in width, about one-thirtieth the diameter of a human hair. In these confined conditions the team observed a striking reorganization of mitochondria in cultured HeLa cancer cells: organelles moved to surround and even indent the nucleus, forming a tight halo the authors named nucleus-associated mitochondria (NAMs).
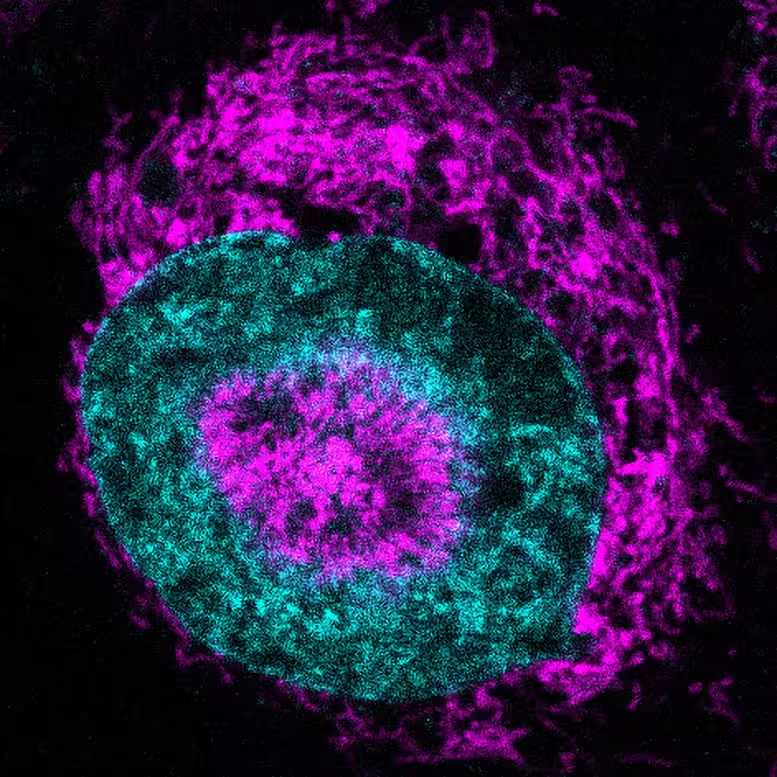
A confined cancer cell, where the mitochondria (in magenta) can be seen accumulating at the nuclear periphery (nucleus shown in cyan) and within nuclear ‘dimples’ (indentations). Credit: Rito Ghose and Fabio Pezzano/Centro de Regulación Genómica
To test whether this repositioning affected nuclear energy levels, the team used a fluorescent ATP sensor targeted to the nucleus. Within seconds of compression, the nuclear ATP signal rose by roughly 60 percent. The surge occurred in more than 80 percent of confined HeLa cells but was essentially absent in non-compressed, floating cells, indicating a rapid, mechanically triggered metabolic response.
Why nuclear ATP matters
Mechanical deformation of the nucleus creates physical strain on chromatin and DNA. DNA double-strand breaks and topological stress require ATP-dependent repair machineries — enzymes and chromatin remodelers that need energy to access and reseal damaged DNA. The study showed that cells experiencing the NAM-driven ATP increase repaired DNA lesions within hours and continued dividing, whereas cells in which this response was blunted failed to resolve damage and showed defective proliferation.
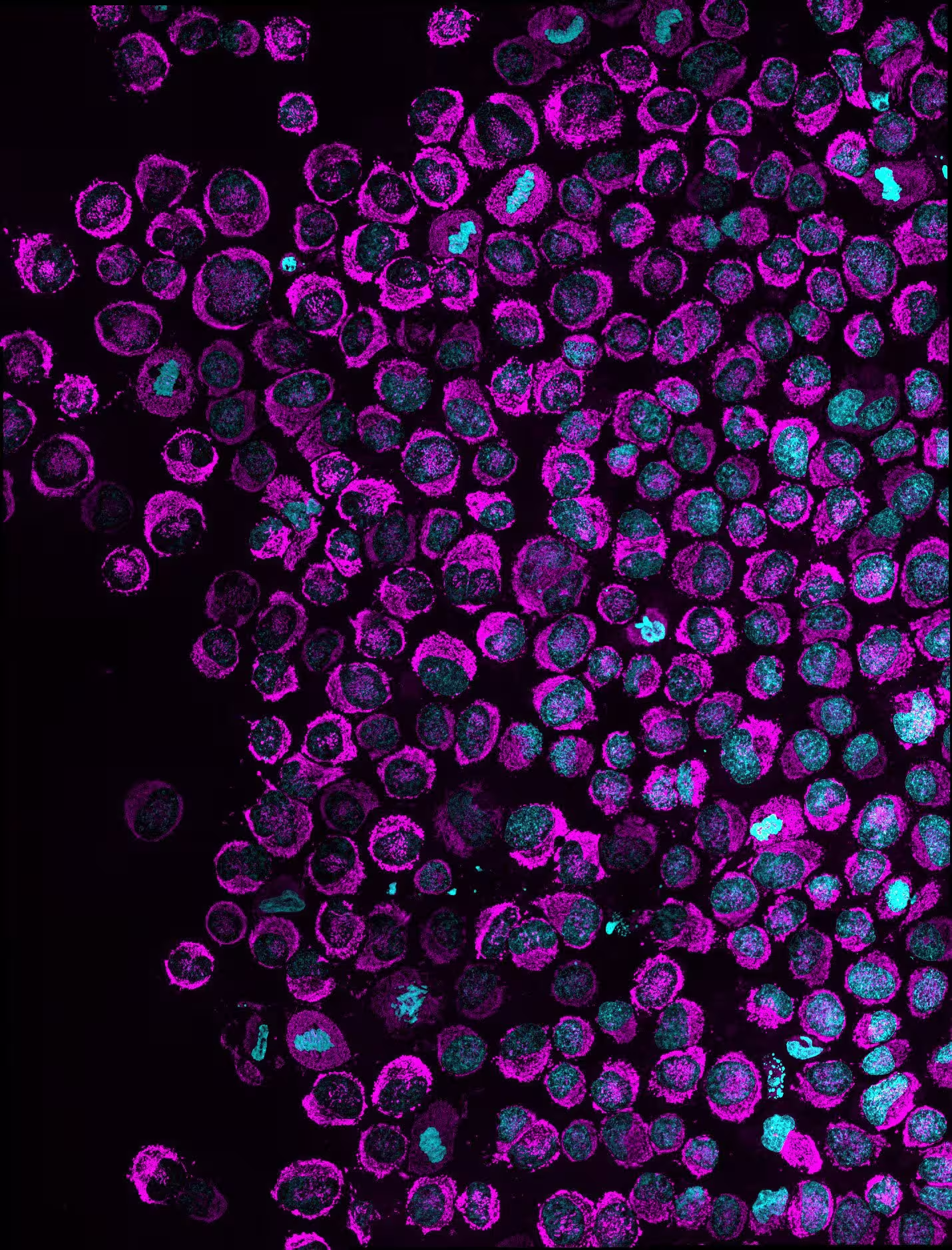
An overview of multiple confined cancer cells, showing mitochondria (in magenta) and the nucleus (in cyan). Cells where you can see the accumulation of mitochondria within the nucleus, show cells with the NAM phenomenon. In HeLa cells (shown in this picture) the penetrance of the phenotype in vitro was 83.6%. Credit: Rito Ghose and Fabio Pezzano/Centro de Regulación Genómica
Cellular scaffold that forms NAMs and pharmacological tests
The authors mapped the cytoskeletal and membrane structures that enable mitochondria to accumulate around the nucleus. Actin filaments compacted near the nuclear envelope while the endoplasmic reticulum (ER) created a mesh that together trapped mitochondria at the nuclear surface. Disrupting actin with latrunculin A dismantled the scaffold, prevented NAM formation and erased the ATP surge, implicating the cytoskeletal network and ER–mitochondria contacts in the process.
This mechanistic insight raises a therapeutic possibility: rather than inhibiting mitochondrial function systemically (which would harm healthy tissues), drugs that selectively impair the scaffold or the mitochondrial tethering that drives NAM formation might reduce cancer cell resilience to mechanical stress and limit invasion.
Evidence from patient samples and disease relevance
To test clinical relevance, the team analyzed breast tumor biopsies from 17 patients. NAM-like mitochondrial halos were more frequent at invasive tumor fronts (5.4% of nuclei) than in the dense tumor core (1.8%), a roughly threefold enrichment consistent with a role in facilitating invasion. While the overall fraction of positive nuclei in tissue is small, the spatial enrichment at invasive fronts supports the idea that NAMs are engaged where mechanical challenges are greatest.
The authors emphasize that the mechanism is not necessarily limited to cancer. Many cell types face mechanical constraints — immune cells squeezing through lymph nodes, migrating neurons, or cells shaping embryos — and may use similar local ATP delivery to preserve genome integrity under stress.
Implications for cancer metastasis and therapy
This discovery reframes mitochondria as dynamic responders that can be rapidly mobilized to sites of highest need, rather than passive cellular batteries. For oncology, the implications are twofold: first, NAM-driven ATP pulses may help circulating or invading cancer cells survive the mechanical rigors of metastasis; second, the molecular machinery that generates NAMs represents a candidate vulnerability. Targeting actin-driven scaffolds, ER–mitochondria contact sites, or the signaling that triggers mitochondrial relocation could reduce metastatic competence with less toxicity than global mitochondrial inhibitors.
Lead authors and collaborators highlighted the conceptual shift. “It forces us to rethink the role of mitochondria in the human body. They aren’t these static batteries powering our cells, but more like agile first responders that can be summoned in emergency situations when cells are literally pressed to the limit,” said Dr. Sara Sdelci, co-corresponding author. Co-first author Dr. Fabio Pezzano added: “It’s a clear sign the cells are adapting to the strain and rewiring their metabolism.”
Co-first author Dr. Ritobrata (Rito) Ghose noted that the presence of NAMs in patient biopsies strengthened the clinical relevance of the finding: seeing the signature outside the lab convinced the team that the phenomenon matters in tumors. Co-corresponding author Dr. Verena Ruprecht suggested that mechanical stress responses are an underexplored vulnerability in cancer biology that could open new therapeutic avenues.
Expert Insight
"This work elegantly links mechanical forces to subcellular energy redistribution and DNA repair," says Dr. Elaine Morgan, a cell biologist (fictional expert) who studies cytoskeletal mechanics. "If we can define the molecular tethers that anchor mitochondria during confinement, it may be possible to design small molecules or biologics that selectively block that relocation in tumor cells without disrupting systemic metabolism. That would be a major advance for anti-metastatic therapy."
Conclusion
The discovery of nucleus-associated mitochondria (NAMs) and their rapid ATP delivery under mechanical confinement reveals a new layer of cellular adaptation. By supplying energy directly to the nucleus, mitochondria help maintain genome integrity during physical stress — a capability that cancer cells exploit to survive and invade. Mapping the structural and signaling components of this response identifies candidate targets to limit metastasis while sparing normal tissue. Further work will be needed to determine how general NAM formation is across cell types and whether selective inhibitors can be developed that safely block this mechanical survival switch in cancer.
Source: scitechdaily

Leave a Comment