5 Minutes
Researchers have developed a fluorescent molecular probe that lights up when marine sugars are broken down, allowing scientists to observe in real time which microbes eat specific carbohydrates and how that feeding shapes carbon storage in the ocean. The tool offers a new, visual approach to tracking microscopic interactions that influence global carbon flux.
How the glowing probe exposes hidden microbial meals
At the heart of this advance is a cleverly engineered sugar molecule tagged with two fluorescent dyes. When the sugar is intact, the dyes suppress visible emission through Förster resonance energy transfer (FRET), keeping the probe effectively dark. Once an enzyme—produced by bacteria or other microbes—cleaves the sugar chain, the FRET interaction breaks and the molecule begins to glow. That glow pinpoints where and when sugar degradation occurs.
Imagine following a bloom of algae and watching, frame by frame, which cells switch on fluorescent signals as they digest complex polysaccharides. That’s the capability the research team demonstrated by tracking α-mannan, a polysaccharide commonly released during algal events. The probe responded to enzyme activity across controlled systems: purified enzymes, bacterial extracts, isolated cultures and mixed microbial communities sampled from seawater.
Why this matters for the ocean carbon cycle
Sugars produced by phytoplankton are a major fraction of organic carbon in the surface ocean. But not all sugars are easily eaten. Some polysaccharides are structurally complex and require specialized enzymes—found in only a subset of microbes—to be degraded. When such sugars resist decomposition, they can sink, transporting carbon to the deep sea where it may be sequestered for decades to centuries.
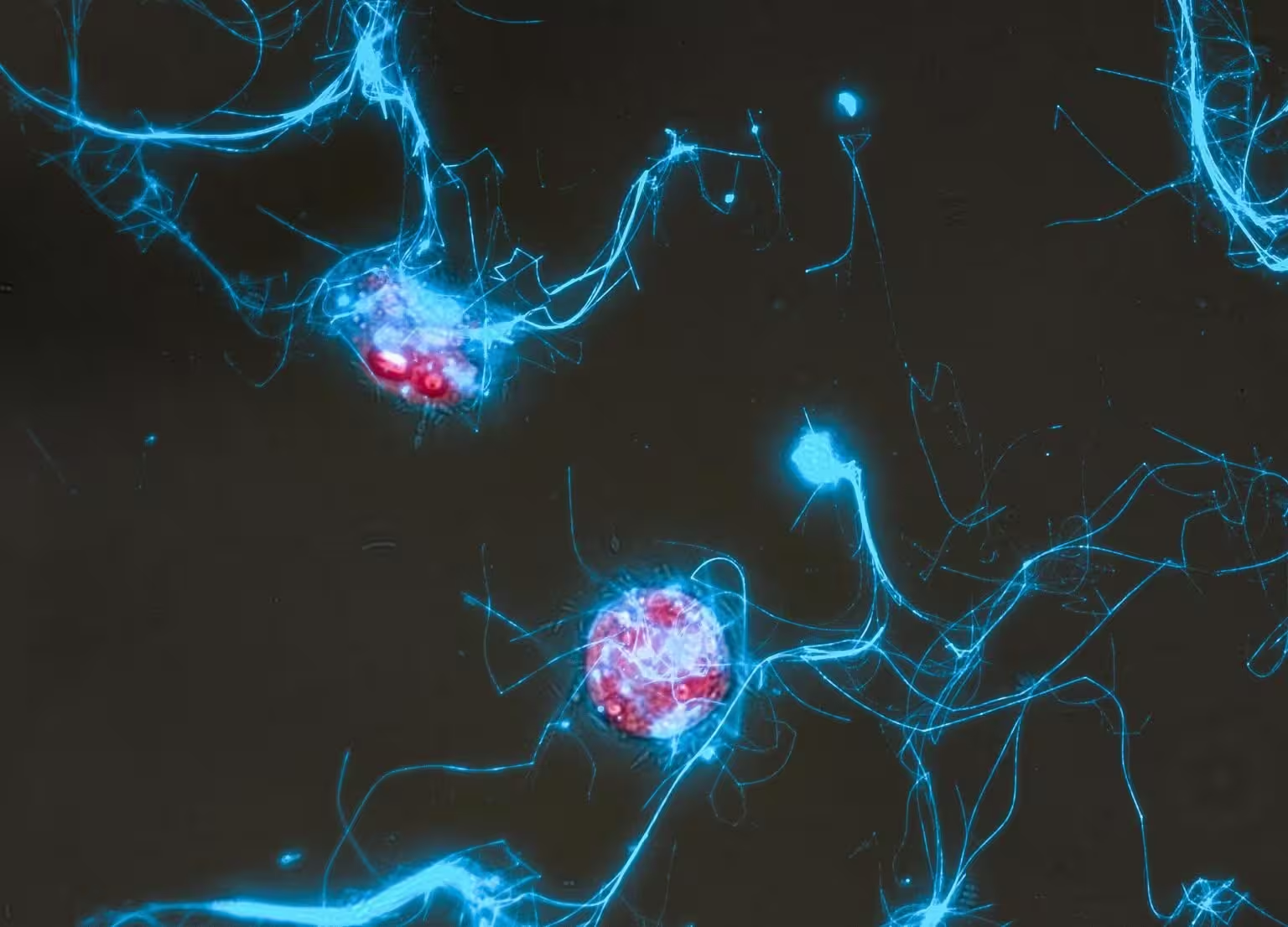
Until now, linking a specific microbe to the turnover of a particular sugar in situ has been difficult because natural communities are diverse and many microbes are uncultured. The fluorescent glycan probe sidesteps this limitation by directly reporting enzymatic activity in the environment without needing prior genomic identification. That means researchers can map turnover rates, identify active degraders, and follow the spatial progression of glycan breakdown in real time—key inputs for models of carbon export and sequestration.
Interdisciplinary engineering: chemistry meets microbiology
The probe was created through automated glycan assembly—a chemical technique that builds defined sugar chains—and precise fluorescent labeling. Experts in colloid and interface science worked with marine microbiologists and ecologists to design the molecule so it would be stable in seawater yet respond promptly when cleaved by relevant enzymes. The team validated the probe’s behavior with laboratory enzymes first, then in living microbial cultures and complex community samples, confirming its robustness across scales.
Beyond α-mannan, the approach is extensible: by synthesizing different glycan structures and attaching appropriate FRET dye pairs, researchers can create probes tailored to other polysaccharides found in marine and terrestrial systems. That adaptability opens doors to studying glycan cycling from algal blooms to soil and even the human gut.
Implications for research and climate science
Quantifying exactly who degrades which sugars—and under what environmental conditions—improves our mechanistic understanding of carbon pathways in the ocean. These insights can refine biogeochemical models that estimate how much carbon is retained near the surface versus how much sinks to depth. More accurate microbial process data will feed into larger-scale climate predictions by constraining one of the ocean’s microscopic but influential carbon valves.
The new method also accelerates ecological discovery. Instead of inferring activity from gene presence, scientists can directly visualize functional enzyme activity and then pair that observation with genomic or imaging tools to identify the active organism. This practical advantage streamlines efforts to link metabolism with taxonomy in complex natural communities.
Expert Insight
"A visual probe like this changes the game for marine microbial ecology," says a senior microbial ecologist familiar with the work. "It lets us ask precise questions: which taxa respond to an algal bloom, how rapidly do they mobilize stored carbon, and how far does that carbon travel before it’s consumed? These are the process-level details models need."
Looking ahead, researchers plan to expand the library of FRET-tagged glycans, deploy probes during field cruises and combine fluorescence imaging with single-cell genomics and mass spectrometry. Together, these tools will sharpen our picture of microscopic food webs and the molecular routes that control carbon movement through the ocean.
Source: scitechdaily
Comments
atomwave
Seems neat but how specific is the probe really? will it misreport other enzymes, or give false positives in messy seawater? curious but skeptical.
bioNix
wow this is wild, watching microbes light up as they eat sugars? kinda mindblowing. if they scale this in the field we might finally see carbon paths in action…


Leave a Comment