8 Minutes
Researchers from Spain, China and the UK report a novel nanotechnology that reversed Alzheimer’s-like symptoms in mice by repairing the brain’s blood–brain barrier rather than targeting neurons directly. The treatment uses specially engineered nanoparticles that act as therapeutic agents—"supramolecular drugs"—to restore vascular clearance of toxic proteins and recover cognitive function in animal models.
Rethinking Alzheimer’s: fix the brain’s plumbing, not the neurons
For decades, Alzheimer’s research has focused on neurons and synapses: preventing neuron death, blocking amyloid formation or clearing tau tangles. This new study adopts a different strategy. Instead of delivering a drug payload to neurons, the team engineered nanoparticles that themselves engage vascular receptors and reset the brain’s waste-clearance machinery. The result: rapid removal of amyloid‑β (Aβ) and a long-term recovery of cognitive behavior in treated mice.
Why target blood vessels? The brain’s vascular network and its protective interface—the blood–brain barrier (BBB)—are essential to brain health. The BBB controls what enters and exits the brain and supports active clearance of metabolic waste. When this system falters, toxic proteins such as amyloid‑β accumulate, inflammation follows, and neuronal circuits degrade. Repairing the BBB restores a core housekeeping function that can halt—or even reverse—disease progression.
Vasculature matters: how the brain keeps itself clean
The brain is extraordinarily energy-hungry. In adults it consumes roughly 20% of the body’s energy and in children that proportion is even higher. This demand is met by a dense web of capillaries—so dense that each neuron is effectively paired with its own tiny blood supply. With nearly a billion capillaries, the brain’s vascular system both feeds neurons and clears waste. When vascular transport breaks down, clearance of harmful proteins stalls and neurodegeneration accelerates.
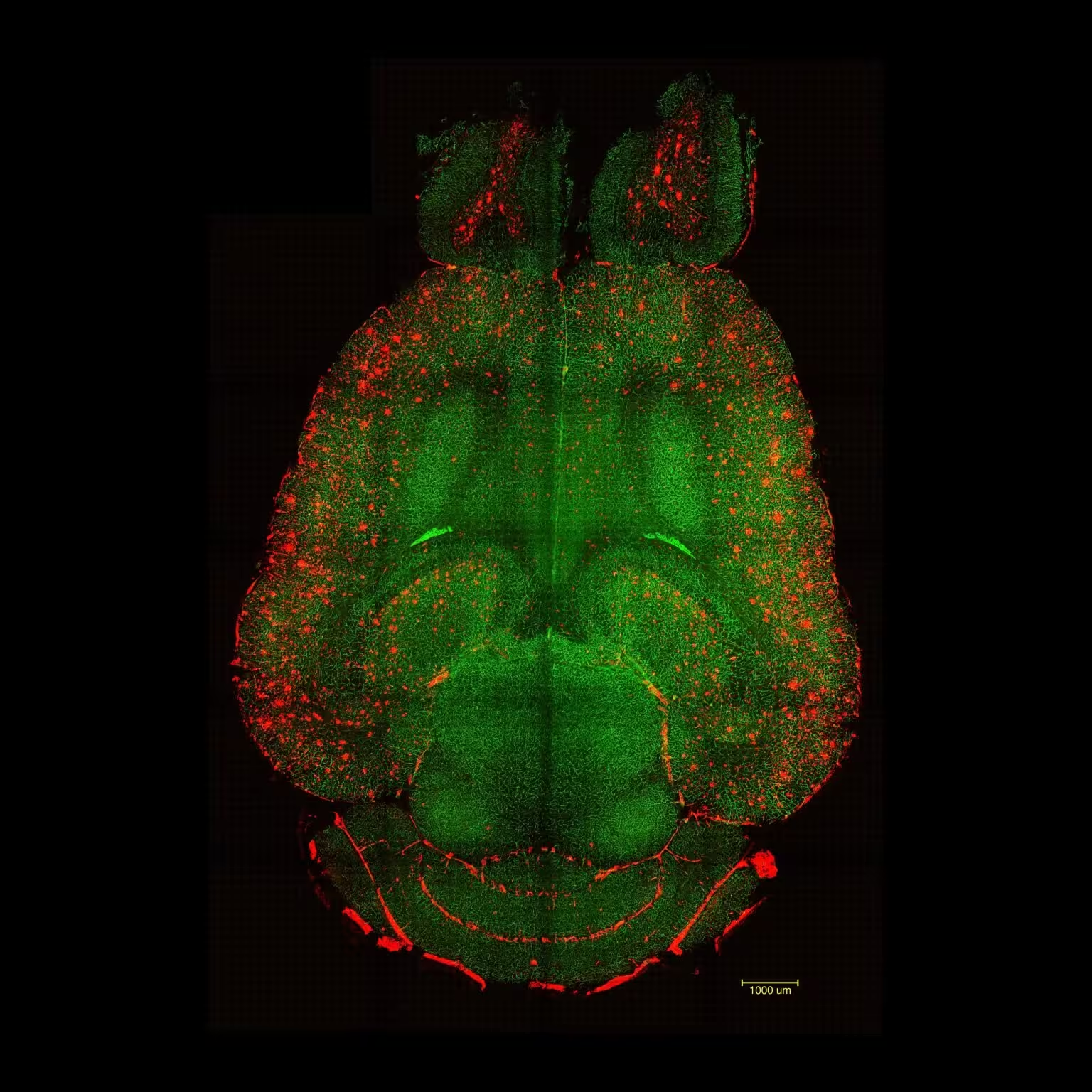
Light sheet fluorescence microscope images of mouse brain 12h after NOT being treated with nanoparticles. The brains were analyzed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)
How the supramolecular nanoparticles work
The team designed nanoparticles as multivalent supramolecular constructs. Rather than acting as passive carriers, these nanoparticles present a defined number of surface ligands that interact with specific receptors on BBB endothelial cells. One key molecular player is LRP1, a receptor that normally binds Aβ and transports it from brain tissue into the blood. In Alzheimer’s conditions, LRP1 trafficking and function are disrupted—either by receptor overload or by weak signaling—so Aβ clearance stalls.
These engineered nanoparticles mimic the natural ligands of LRP1 and modulate receptor trafficking: they bind Aβ, engage LRP1, and prompt the receptor to ferry the toxic cargo across the BBB. In effect, the nanoparticles act like a biochemical switch that resets the clearance pathway and reactivates vascular waste removal. Because the particles are therapeutic agents themselves, the approach sidesteps issues associated with drug-loading and release kinetics common to traditional nanomedicine.
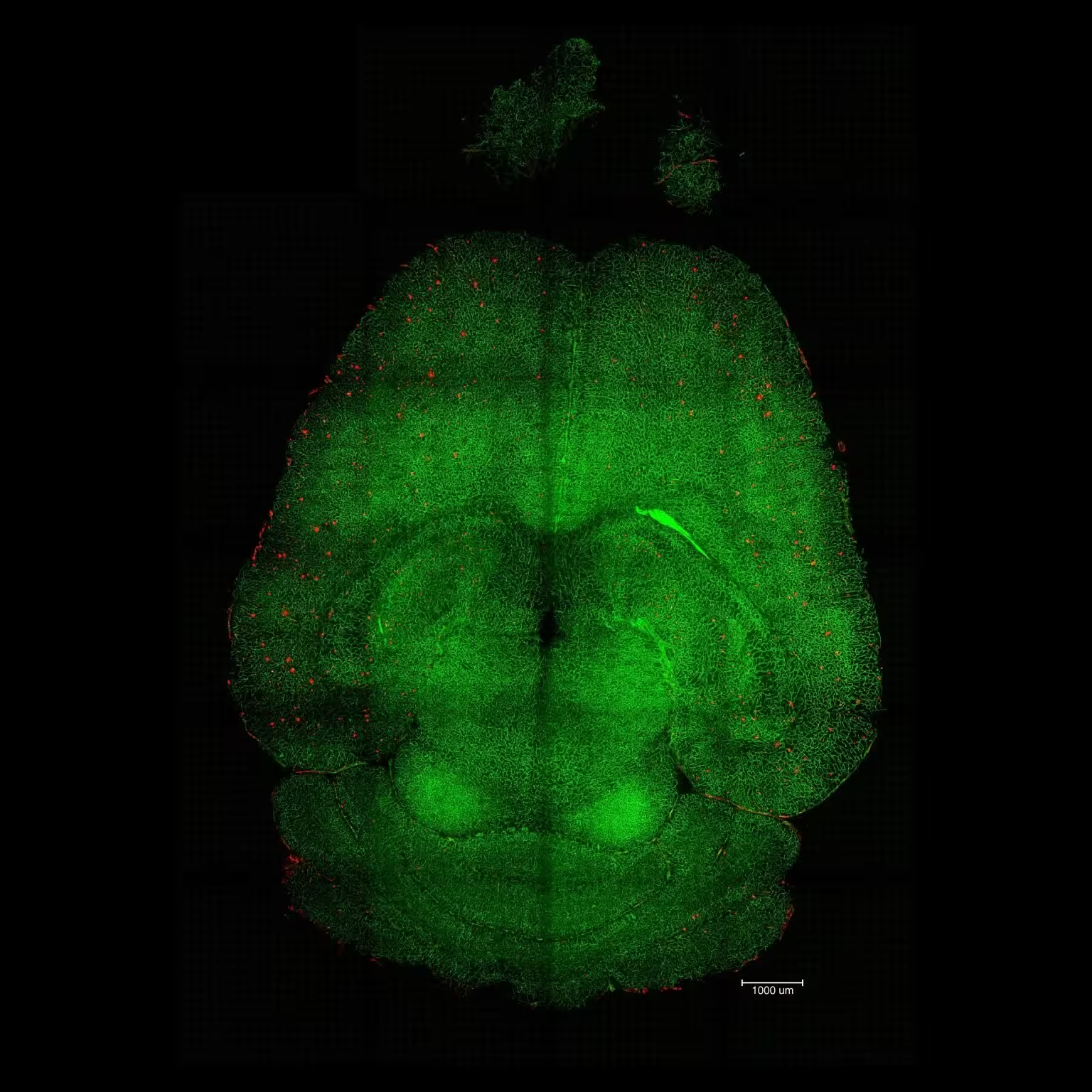
Light sheet fluorescence microscope image of mthe ouse brain 12h after being treated with nanoparticles. The brains were analyzed to see the amount of Aβ plaque accumulation. Red: Aβ plaques. Green: vessels from the blood-brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)
Fast-acting and durable effects in Alzheimer’s mice
To test efficacy the researchers used genetically engineered mice that overproduce Aβ and show progressive cognitive decline. Treatment consisted of just three injections of the supramolecular nanoparticles. The clearance was both fast and profound: within one hour of injection investigators observed a 50–60% reduction in measurable Aβ inside the brain.
But the most remarkable outcomes were behavioral and long-lasting. Animals were assessed across multiple disease stages and over months. In one experiment a 12‑month‑old mouse (roughly equivalent to a 60‑year‑old human) received the nanoparticles and was retested six months later. The treated 18‑month‑old animal (comparable to a 90‑year‑old human) displayed behavior indistinguishable from healthy controls. The authors attribute these durable benefits to restored vascular function: once clearance pathways reboot, Aβ and other toxic species are progressively removed and the whole system moves back toward equilibrium.
"Only 1h after the injection we observed a reduction of 50-60% in Aβ amount inside the brain," explained Junyang Chen, a first co-author and researcher at West China Hospital of Sichuan University. The team emphasizes that the nanoparticles act via vascular modulation and receptor trafficking rather than direct neuronal rescue.

Giuseppe Battaglia (left) and Lorena Ruiz Pérez (right). Credit: Institute for Bioengineering of Catalonia (IBEC)
Scientific context: LRP1, receptor trafficking and multivalency
LRP1 (low-density lipoprotein receptor-related protein 1) functions as a molecular gatekeeper for Aβ. It recognizes the peptide, binds through accessory ligands, and mediates transcytosis across endothelial cells. The trafficking of LRP1 is finely balanced: overly tight binding or receptor degradation can deplete transport capacity, while weak engagement fails to trigger transit. The supramolecular nanoparticles exploit this balance by presenting multiple ligand copies (multivalency) to produce an optimal engagement profile that activates receptor-mediated clearance without clogging the system.
Engineering precision matters: by controlling particle size and the number of surface ligands, the research team achieved a platform that modulates receptor behavior at the cell membrane—opening a new route to treat vascular contributions to dementia.

Xiaohe Tian (left) and Giuseppe Battaglia (right). Credit: Institute for Bioengineering of Catalonia (IBEC)
Potential clinical implications and challenges ahead
The findings suggest a different therapeutic axis for Alzheimer’s disease—one that targets vascular health and waste clearance rather than attempting to rebuild damaged neurons directly. Restoring BBB function could complement other strategies, including anti‑Aβ antibodies or tau-targeted therapies, and might be especially valuable in mixed-pathology cases where vascular dysfunction contributes heavily to symptoms.
However, translating mouse success into human benefit faces hurdles. Safety profiles, off-target effects, dosing regimens, manufacturing scale-up and long-term immunogenicity must be evaluated. The BBB is also more complex in humans, and individual variability in LRP1 expression or vascular pathology could influence therapeutic windows. Carefully designed preclinical safety studies and early-phase clinical trials will be essential.
"The long-term effect comes from restoring the brain’s vasculature," said Lorena Ruiz Perez of IBEC, noting that the nanoparticles appear to trigger a feedback mechanism that reactivates natural clearance pathways. While promising, she and colleagues emphasize the need for rigorous translational work before human use.
Related technologies and future prospects
This work sits at the intersection of molecular bionics, supramolecular chemistry and neurovascular biology. Similar multivalent constructs are being explored for targeted immunomodulation, receptor reprogramming and as synthetic ligands to direct cell-signaling. If safety and efficacy translate to humans, supramolecular nanoparticles could become a modular platform for other neurological conditions where clearance and vascular function are compromised, such as small‑vessel disease or some forms of frontotemporal dementia.
Expert Insight
Dr. Maya Thompson, a neurologist and vascular neurobiology researcher not involved in the study, commented: "Targeting the blood–brain barrier is an elegant pivot. Many neurodegenerative disorders have a vascular component that’s been underappreciated. These supramolecular particles are exciting because they act on the brain’s cleanup crew—if we can prove safety and reproducibility in larger models, this approach could reshape how we design therapies for dementia."
What this means for patients and science communication
For patients and families, the study offers a hopeful signal: brain recovery may be possible by restoring natural systems that already exist rather than trying to force cell regeneration. For scientists, it reinforces the importance of integrated approaches that consider neurovascular health alongside neuronal biology.
Source: scitechdaily
Comments
Reza
Feels overhyped but clever. Resetting LRP1 trafficking is neat, just not convinced scaling, dosing and long term immunogenicity wont be a headache. Hope I'm wrong.
bioNix
Is this even true? Three injections, 50% drop in Aβ in 1 hour sounds almost too good. BBB is way more complex in humans, so show me primate data first, curious though.
mechbyte
Wow, didnt expect blood vessels to be the hero here... if mice really translate to people this could be massive. Fingers crossed, but safety + immune reactions worry me, tbh


Leave a Comment