3 Minutes
Paradromics, a pioneering brain-computer interface (BCI) startup, has achieved a significant breakthrough by successfully testing its Connexus brain implant in a human subject. The short-term test marks an important step forward for clinical neurotechnology and the rapidly evolving field of brain-computer interfaces, positioning Paradromics as a key rival to industry leader Neuralink.
Background: The Growing Race in Brain-Computer Interfaces
Brain-computer interfaces (BCIs) are advanced systems that decode neural signals and translate them into commands for external devices, with the potential to restore lost abilities or enhance human-machine interactions. Companies like Neuralink and Paradromics are at the forefront of this innovation, aiming to improve the lives of patients with severe neurological conditions.
The Connexus Implant and Its First Human Test
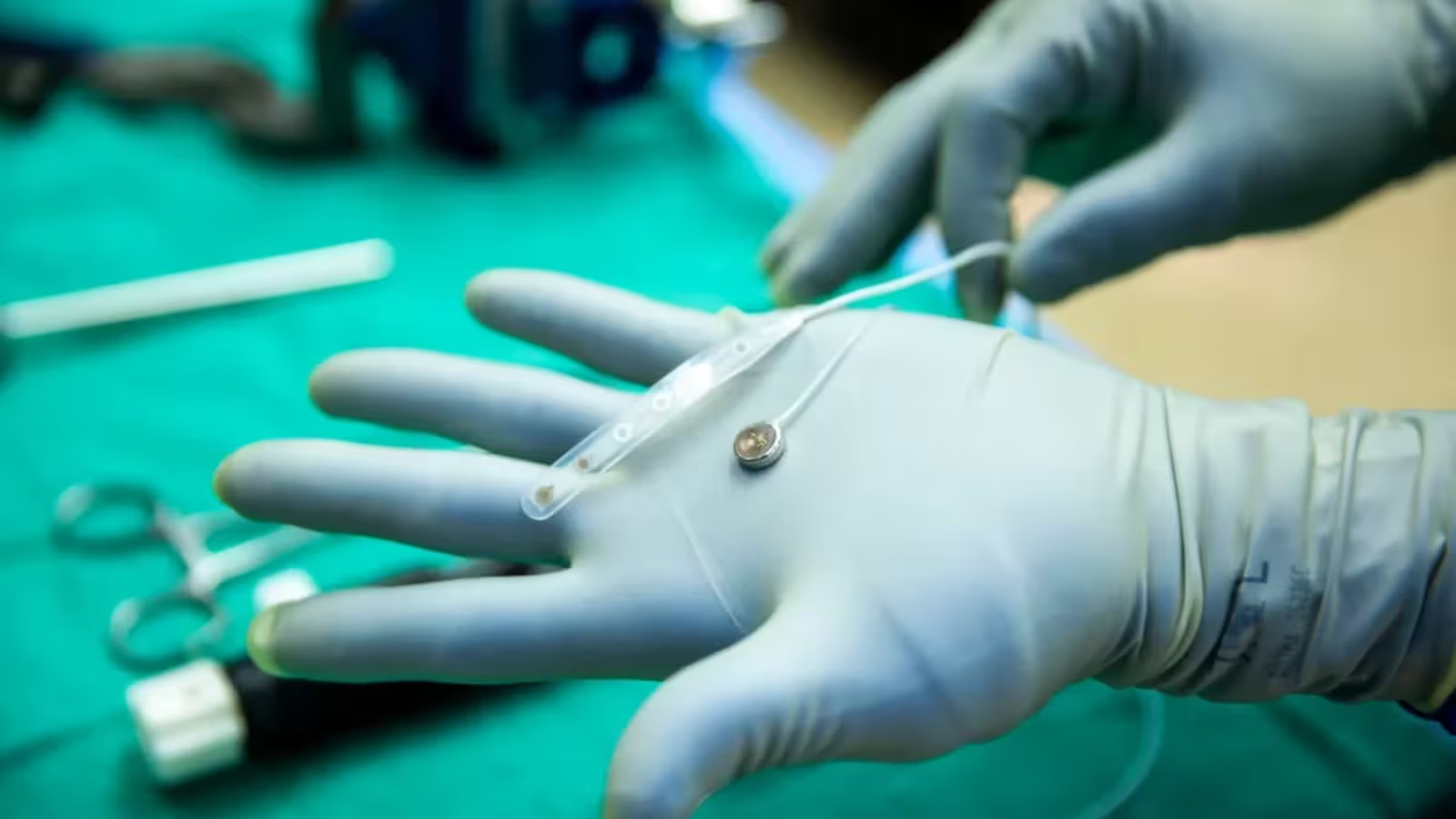
According to a report by Wired, Paradromics conducted the first human test of its Connexus implant on May 14 at the University of Michigan. The procedure involved a volunteer who was already undergoing brain surgery for epilepsy treatment. The Connexus implant was inserted into the patient’s brain for ten minutes to assess safety and functionality, and then removed without complications.
Paradromics announced that during this short-term placement, the device was able to safely record neural activity—a key indicator of both the system’s safety and technological readiness. This demonstration paves the way for more extensive, long-term clinical trials that will explore the implant’s durability and effectiveness in real-world use.

Expert Insight and Future Clinical Trials
Paradromics’ founder and CEO, Matt Angle, shared with CNBC: “In our animal studies, notably with sheep, we’ve proven that our device leads the field in both data performance and durability. Now, we’ve shown it is compatible with the human body as well.”
The company plans to begin larger clinical trials by late 2025, pending regulatory approvals. These studies will evaluate the Connexus system’s long-term safety and practical benefits for patients, specifically targeting individuals with severe motor disabilities who could communicate via computers using the implant.
Competitive Landscape and Commercial Prospects
While Paradromics is steadily progressing, its brain implant technology still awaits approval from the US Food and Drug Administration (FDA). Commercialization is anticipated to be several years away, as rigorous safety and efficacy benchmarks must be met. Paradromics recently announced collaborations in brain chip development, including partnerships in the Middle East.
Neuralink, Paradromics’ main competitor, has already implanted its BCI chips in three human patients, with notable results such as enabling a paralyzed individual to communicate through the system. As the competition heats up, both firms are driving the industry toward transformative applications in treating neurological disorders and restoring lost functions.
Conclusion
Paradromics' successful short-term test of its Connexus brain implant marks a crucial advance in brain-computer interface technology. As clinical trials expand and regulatory review progresses, the company could play a major role in shaping a future where BCIs restore independence and communication for patients with neurological disabilities. The race between Paradromics and Neuralink is set to accelerate innovation and knowledge in the neuroscience and medical technology sectors.
Source: wired


Comments