5 Minutes
Researchers report a first-in-human stem-cell transplant that appears to restore central vision in some older adults with dry age-related macular degeneration (AMD). Early-phase clinical data show the procedure is safe and produced meaningful vision gains in the most severely affected trial participants, prompting further testing at higher doses.
Why central vision fades in dry AMD — and why it matters
Age-related macular degeneration is the leading cause of irreversible central vision loss among older adults. The "macula" is the small, central part of the retina that enables tasks that require sharp focus — reading, recognizing faces, and driving. In the dry form of AMD, which represents roughly 80% of cases, tiny deposits of fats and proteins called drusen accumulate beneath the retinal pigment epithelium (RPE). Over time these deposits damage RPE cells, the support tissue that nourishes light-sensitive photoreceptors, and this progressive cell loss blurs the central visual field.
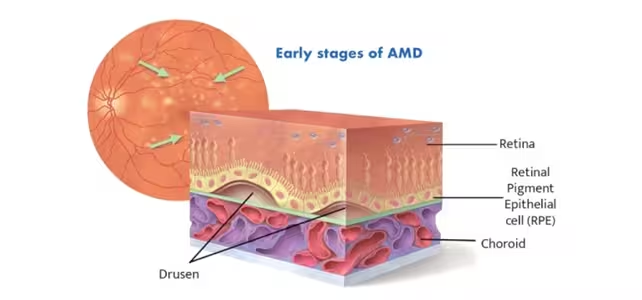
Diagram showing early stages of dry macular degeneration, caused by deposits of fats and proteins known as drusen beneath the RPE.
What the new trial tested and how the procedure works
The published phase 1/2a study evaluated an RPE cell transplant derived from adult stem cells supplied by an eye bank. The goal at this stage was safety and preliminary efficacy — not yet a head-to-head comparison with existing therapies. Investigators screened 18 potential participants and enrolled six volunteers aged 71 to 86 who had advanced dry AMD in one eye.
Delivery and dosing
Each participant received a single, relatively low dose of 50,000 RPE stem cells, injected under the retina into the superior temporal macula of the eye with worse vision. The transplanted cells are intended to replace damaged RPE tissue and restore the microenvironment that keeps photoreceptors healthy.
Early lab work and safety checks
Preclinical laboratory studies had shown the transplanted cells retained their retinal identity and did not generate tumors or systemic toxicity — findings that paved the way for human testing. During the trial, researchers monitored participants closely for immune reactions, tumor formation, and the common complications associated with retinal surgery.
Key results: safety first, plus unexpected vision gains
The trial met its primary safety endpoints. There were no adverse events directly attributed to the implanted stem cells — no tumor formation and no evidence of immune-related rejection linked to the cells themselves. Some participants did experience typical surgical complications, but these were manageable and unrelated to the cell product.
On the efficacy front, the results that surprised investigators were improvements in vision limited to the treated eye. Three participants with the poorest baseline acuity (from roughly 20/200 to 20/800) gained, on average, 21 letters on an eye chart one year after the injection. The other three participants, who began with better acuity (roughly 20/70 to 20/200), showed smaller improvements. The unilateral improvement — only in the eye that received cells — strengthened the case that the transplant itself contributed to the visual gains.
"Although we were pleased with the safety data, the exciting part was that their vision was also improving," said Rajesh Rao, physician-scientist and ophthalmologist at Michigan Medicine. "We were surprised by the magnitude of vision gain in the most severely affected patients who received the adult stem cell-derived RPE transplants. This level of vision gain has not been seen in this group of patients with advanced dry AMD."
What this means for patients and next steps in research
These findings are promising but preliminary. The small sample size (six participants at the low dose) means larger randomized trials are needed to confirm benefit, measure durability, and compare outcomes with existing or emerging treatments. The trial continues to follow groups receiving higher doses — 150,000 and 250,000 cells — to determine whether scaling the dose maintains safety and enhances efficacy.
If higher doses are safe and produce equal or greater vision recovery, researchers will design phase 3 trials to test the treatment against the current standard of care. Even then, regulatory review, manufacturing scale-up, and accessibility for patients will take additional years.
Related technologies and clinical context
Stem-cell–derived RPE transplants belong to a growing suite of regenerative approaches targeting retinal disease, from gene therapies that correct molecular defects to cell-sheet implants and drug-delivery strategies designed to slow degeneration. Together, these approaches aim to either preserve remaining vision or restore function once cells are lost.
For patients with dry AMD — who currently have limited options beyond lifestyle changes and low-vision aids — a safe, effective RPE transplant could shift treatment from mitigation to partial restoration.
Expert Insight
"A targeted RPE transplant addresses a specific failure point in dry AMD: the loss of supportive pigment epithelial cells," says Dr. Elena Morales, a retinal surgeon and translational scientist. "If we can reliably replace those cells without triggering immune problems or tumor risks, the downstream benefit to photoreceptors could be substantial. The next hurdles are reproducibility and long-term integration of the grafted cells into the complex retinal architecture."
Source: sciencealert
Comments
mechbyte
Promising but only six ppl, that's tiny. 50k cells feels low, curious about dose scaling and immune suppression longterm. Is this really scalable?
labcore
Wow, real stem cells restoring central sight? If that holds up this could change lives. Hoping they follow patients long term though, fingers crossed...

Leave a Comment