5 Minutes
Researchers in Japan report that oral arginine — an amino acid already used for chest pain and high blood pressure — cleared amyloid-beta protein aggregates in mice and fruit flies. The finding, published in Neurochemistry International, suggests a low-cost, clinically safe candidate for further testing against a core molecular feature of Alzheimer’s disease.
Why arginine could target protein clumps
Amyloid-beta plaques are sticky protein aggregates long associated with Alzheimer's pathology. They accumulate between neurons and are linked to synaptic dysfunction and cell death. Arginine has been known to act as a chemical chaperone — a small molecule that helps proteins fold correctly or prevents them from misfolding and sticking together.
That property is central to the new study’s premise: if arginine can both dissolve existing aggregates and stop new clumps from forming, it may reduce one of the most visible molecular hallmarks of Alzheimer’s. Crucially for brain therapies, previous work has shown arginine can cross the blood-brain barrier, a major hurdle for neuroactive drugs.
Study setup: animals, doses and outcomes
A team from Kindai University and Japan’s National Institute of Neuroscience bred male mice engineered to develop Alzheimer’s-like amyloid-beta aggregations. Instead of injections or invasive delivery, researchers added arginine to the animals’ drinking water — an oral route that mirrors how patients might take a future therapy.
Alongside the mouse experiments, the researchers ran complementary tests in fruit flies and in vitro assays to map arginine’s effects on amyloid-beta aggregation more directly. These parallel approaches helped separate systemic effects (for example, changes in inflammation) from direct actions on protein clumps.
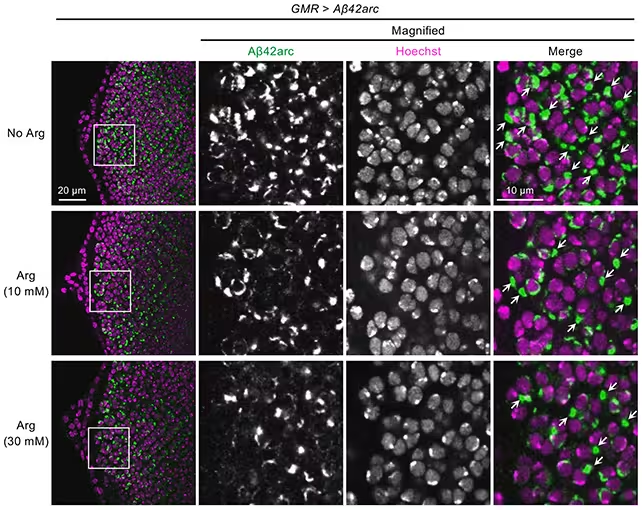
Arginine reduced amyloid-beta clumping (in green) in fruit fly models.
Key findings and biological signals
Mice receiving arginine showed significantly less amyloid deposition in the brain and displayed smaller degrees of behavioral abnormalities during standard tests. Molecular analysis also revealed dampened activity in neuroinflammatory genes — suggesting the treatment may do more than just dissolve plaques; it could reduce downstream inflammation that contributes to neuronal damage.
In vitro experiments and the fruit fly work supported a direct anti-aggregation effect: arginine appeared to both clear existing fibrils and limit new amyloid-beta assembly. Taken together, these results argue for a dual-action mechanism: arginine as a chemical chaperone plus a modulator of inflammatory responses.
What this means — and what it doesn’t
Lead neuroscientist Yoshitaka Nagai summed up the promise: 'Our study demonstrates that arginine can suppress amyloid-beta aggregation both in vitro and in vivo. What makes this finding exciting is that arginine is already known to be clinically safe and inexpensive, making it a highly promising candidate for repositioning as a therapeutic option for Alzheimer's disease.'
However, the authors and commentators urge caution. The animal studies used relatively high arginine doses; safe and effective human dosing remains unknown. And while animal and fly models are useful, they are not perfect proxies for human Alzheimer’s. Many interventions that work in mice have failed in clinical trials.
There is also a broader scientific debate: removing amyloid plaques has not consistently translated into cognitive benefit in patients, raising questions about whether plaques are drivers of disease or downstream markers of other toxic processes. Arginine’s additional anti-inflammatory and proteostasis (protein-handling) effects may therefore be as important as plaque clearance itself.
Expert Insight
Dr. Maria Chen, a neuropharmacologist not involved in the study, comments: 'This is an elegant, pragmatic study. The oral route and the known safety profile make arginine a logical candidate for rapid translation into early-phase trials. Still, clinical endpoints must include cognition and biomarkers beyond plaque load to show real patient benefit.'
Next steps include dose-finding and safety evaluations in humans, followed by carefully designed trials that measure cognitive outcomes, amyloid biomarkers and markers of neuroinflammation. If arginine can be shown to shift multiple pathological processes with acceptable safety, it could join a growing portfolio of strategies aimed at protein misfolding disorders.
In short, while arginine is not a proven therapy for Alzheimer’s yet, these animal results provide a clear rationale for the next phase of clinical research — and a reminder that repurposing inexpensive, well-understood molecules can sometimes yield fast routes toward new treatments.
Source: sciencealert
Comments
Marius
Is this even true? Mice+flies are neat, but humans are a whole other story. High doses, unkown safety, lots of gaps. If that's real then…
atomwave
wow, arginine cleared amyloid in mice? kinda hopeful but pls no hype yet… low cost is great though, fingers crossed

Leave a Comment