5 Minutes
Tiny plastic fragments — microplastics and nanoplastics (collectively NMPs) — are increasingly detected in potable tap water worldwide. These particles, derived from polystyrene, polyethylene, polypropylene, polyethylene terephthalate (PET) and other polymers, enter drinking supplies through broken down consumer plastics, wastewater, and treatment-system bypasses. While the full health effects of chronic NMP ingestion remain under investigation, laboratory and animal studies link them to changes in the gut microbiome and potential impacts on immune and antibiotic-resistance pathways. In 2024, researchers from Guangzhou Medical University and Jinan University reported a practical mitigation technique: heating tap water to boiling and then filtering out mineral precipitates that trap plastic particles.
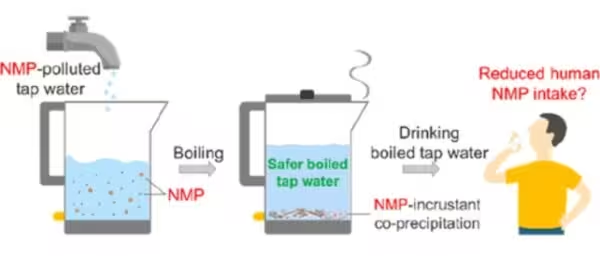
How boiling and mineral precipitation removes microplastics
Experiment design and key measurements
The research team simulated household conditions by spiking both soft and hard tap water with representative nano- and microplastic particles, then boiling the samples and collecting any solids that formed. Hard water contains higher dissolved calcium and bicarbonate. When heated, calcium carbonate (what we commonly see as limescale in kettles) comes out of solution and forms a chalky crust. The investigators quantified how many NMPs co-precipitated with this mineral crust and how many remained in the liquid after boiling and simple filtration.
Results — effectiveness varies with water hardness
Boiling followed by straining removed a substantial fraction of NMPs in many test cases. Removal efficiency rose with water hardness: measured capture of nanoplastics increased from about 34% at 80 mg L−1 calcium carbonate to roughly 84% at 180 mg L−1 and 90% at 300 mg L−1. Even in soft water, with low dissolved calcium, roughly a quarter of the particles were entrained by precipitation. Simple household filters — for example a stainless-steel tea-strainer or a fine mesh sieve — were sufficient to remove the lime-encrusted plastic aggregates from boiled water.

Implications, limitations and context
The major advantage of this approach is accessibility: boiling and a basic mesh filter are available in most kitchens. As the study authors note, traditional practices of boiling drinking water could have an underappreciated benefit in reducing NMP intake. Biomedical engineer Zimin Yu and colleagues describe the method as a "highly feasible strategy" to lower human exposure to drinking-water nanoplastics and lay the groundwork for larger-scale investigations.
However, boiling is not a universal solution. It does not remove dissolved chemical pollutants (such as lead, nitrates, or many organic contaminants) and concentrates any nonvolatile solutes as water volume decreases. Extremely small nanoplastics below detection or particles with different surface chemistries may behave differently. The laboratory tests used spiked samples under controlled conditions; real municipal systems contain complex mixtures of minerals, organic matter, and microbial communities that could influence precipitation and particle capture.
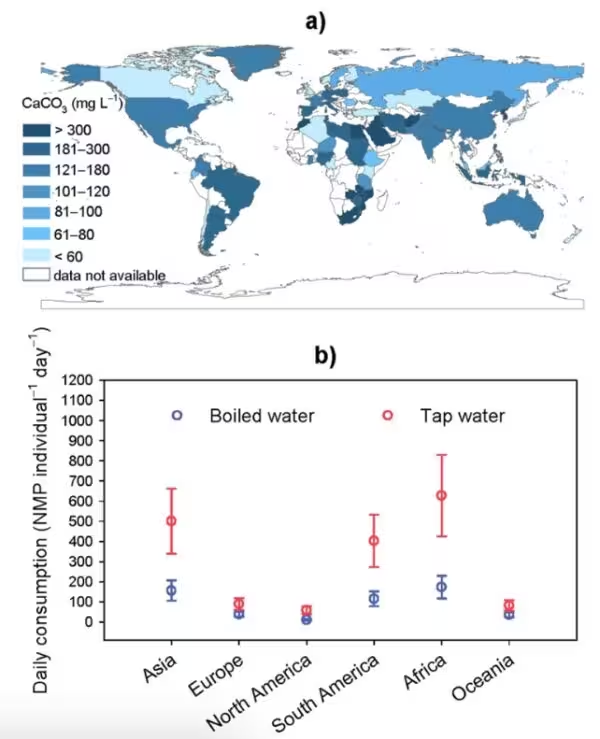
Related technologies and future directions
Point-of-use treatments such as activated carbon filters, ultrafiltration, and reverse osmosis remain effective options for broader contaminant removal and can complement boiling/straining for particle reduction. Future research should expand field trials across diverse water chemistries, measure removal of the smallest nanoplastics, and test long-term impacts on household appliance maintenance (e.g., limescale build-up) and taste. The researchers call for larger sample sets and real-world evaluations to confirm the method's scalability and public-health relevance.
Practical guidance for consumers
If you want to reduce potential microplastic intake from tap water using this method: boil water thoroughly, allow solids to settle if present, then pour through a fine stainless-steel mesh or household strainer to collect visible precipitates and encrusted particles. Remember this is a mitigation measure: it lowers exposure to particulate plastics but does not remove dissolved toxins or replace the need for safe municipal treatment practices.

Conclusion
Laboratory experiments indicate that a simple household habit — boiling tap water and straining out mineral precipitates — can capture and remove a substantial share of micro- and nanoplastics, especially from hard water that forms calcium carbonate scale. The technique is low-cost and widely accessible, offering a pragmatic way to reduce one pathway of human NMP exposure while scientists continue to clarify health risks and develop complementary water-treatment technologies. Further real-world testing and larger datasets will be essential to validate effectiveness across varied water systems and particle types.
Source: pubs.acs


Leave a Comment