6 Minutes
Laboratory Recreation of a Primordial Reaction
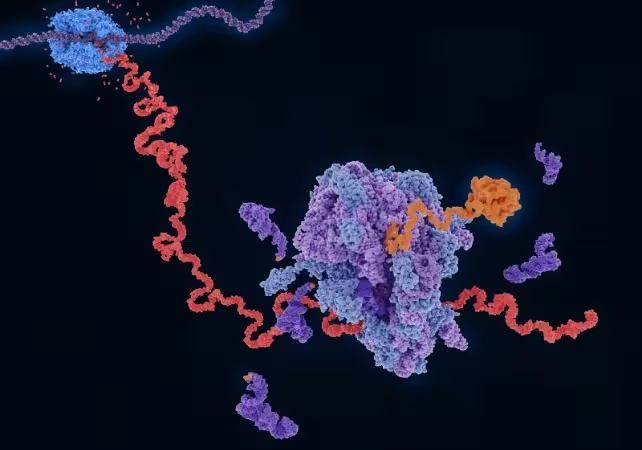
Scientists have reproduced a critical chemical event that may have occurred on Earth about 4 billion years ago: the spontaneous attachment of amino acids to RNA in water. Recreating plausible prebiotic conditions, researchers at University College London demonstrated that activated amino acids can bond to RNA chains using thioesters as the high-energy mediator. This experimental advance bridges major theories about the origin of life and provides a testable pathway for how the molecular partnership between nucleic acids and proteins could have arisen in the planet's early “organic soup.”
Scientific background: RNA world, thioesters, and the problem of energy
The transition from simple organic molecules to living systems requires two central players: nucleic acids (like RNA) that can store and transmit information, and proteins (assembled from amino acids) that perform most cellular functions. Modern biology uses the ribosome — a complex molecular machine guided by messenger RNA (mRNA) — to translate genetic sequences into proteins. But on the prebiotic Earth there were no ribosomes, raising a fundamental question: how did RNA and amino acids first come together to enable primitive peptide formation and eventually give rise to coded protein synthesis?
One influential idea, the RNA world hypothesis, proposes that early life was based on RNA capable of self-replication and catalytic activity. However, joining amino acids to nucleic acids requires an energy-rich activating agent. Many candidate activators degrade in water or cause amino acids to form undesirable byproducts rather than attach to RNA. Thioesters — sulfur-containing, energy-rich compounds — have been proposed as plausible primordial energy carriers in the so-called thioester world hypothesis. Thioesters are chemically reactive but are also known to play central intermediary roles in modern biochemistry, making them compelling prebiotic mediators.
Experiment details: simulating early Earth chemistry
Led by chemist Jyoti Singh, the research team designed aqueous reactions at neutral pH that combined short RNA oligomers with activated amino acids and thioester compounds. The experimental setup deliberately mimicked wet, mild conditions that are more realistic for early Earth than harsh, anhydrous chemistries. Instead of using overly reactive intermediates that rapidly hydrolyze, the team selected thioester activation because it can provide the necessary coupling energy while remaining compatible with water.
Under these conditions the researchers observed that thioesters enabled amino acids to attach selectively to RNA molecules. The chemistry proceeded without extensive external manipulation and appeared both spontaneous and selective — essential attributes for a plausible prebiotic pathway. According to the authors, this mechanism could explain how short peptides began to form in the presence of informational polymers long before modern translation machinery existed.
Key discoveries and implications for origins-of-life research
The primary finding is that thioesters can act as effective, prebiotically reasonable activating groups that facilitate amino acid–RNA linkage in water. This result unites two distinct theoretical frameworks: the RNA world (information and catalysis driven by RNA) and the thioester world (energy flow mediated by thioesters). If early Earth chemistry allowed RNA to selectively receive amino acids via thioester activation, it creates a plausible chemical continuity from nonliving organics to primitive peptide synthesis and, eventually, coded translation.
This experiment stops short of demonstrating the emergence of a genetic code or ribosomal-like translation, but it establishes a critical chemical bridge — the formation of peptide-like chains attached to informational polymers. That bridge is necessary for hypotheses that propose gradual evolution from simple catalytic RNAs to more complex ribonucleoprotein assemblies and ultimately to protein-dominated biology.
Expert Insight
Dr. Helen Ortiz, a molecular evolutionist (fictional for commentary), notes: "This work is important because it demonstrates a water-compatible route for coupling amino acids to RNA. Prebiotic models that rely on dry or extreme conditions face challenges in explaining how early polymers coexisted. Using thioesters provides a chemically reasonable energy source that modern biochemistry also employs, which strengthens the plausibility of this pathway."
Dr. Ortiz adds that the next milestones will be to test whether RNA shows preferences for specific amino acids under these conditions and whether short peptides formed this way can feed back to improve RNA catalysis — essential steps toward a primitive translation system.
Limitations and next steps
The experiments do not yet demonstrate the selective pairing between particular codons and amino acids that underlies the genetic code. Future experiments must determine whether RNA sequences bias which amino acids attach, and whether attached amino acids can form peptides of sufficient length and function to influence RNA replication or catalysis. Researchers will also explore environmental variables such as wet–dry cycles, mineral surfaces, and fluctuating temperatures, which could concentrate reactants and drive more complex chemistry.
Broader significance and future prospects
If validated and extended, these findings could reshape how origin-of-life research conceptualizes energy flow and molecular partnership on the early Earth. The study suggests a coherent narrative for how simple carbon, hydrogen, nitrogen, oxygen, and sulfur building blocks might self-assemble into functional biomolecules. This pathway is not only central to understanding Earth's biogenesis but could also inform the search for life elsewhere by highlighting chemical markers — for example, sulfur-rich activating chemistries — that indicate prebiotic peptide assembly.
The work also intersects with synthetic biology and astrobiology: reproducing prebiotic peptide synthesis in mild, aqueous conditions can guide laboratory efforts to build minimal replicators and inform target chemistries for missions probing icy moons or ancient Martian sediments.
Conclusion
The UCL-led experiments demonstrate a plausible, water-compatible mechanism for linking amino acids to RNA using thioester activation. By combining elements of the RNA world and thioester world hypotheses, the research provides an experimentally grounded route toward the earliest steps in protein synthesis and peptide formation. While many challenges remain — notably, establishing selective amino acid attachment that could lead to a genetic code — this study marks an important advance in reconstructing the chemical origins of life and sets clear directions for future investigations into prebiotic chemistry and early molecular evolution.
Source: nature


Leave a Comment