5 Minutes
Study summary and context
A team at Washington University School of Medicine in St. Louis reports that lemborexant, an FDA-approved sleep medication, restored healthier sleep patterns and reduced tau-driven brain damage in mouse models of neurodegeneration. The peer-reviewed study, published in Nature Neuroscience, links improved sleep to lower levels of abnormal tau protein, reduced neuroinflammation, and less neuronal loss in mice predisposed to tau accumulation.
A familiar, FDA-approved sleep aid restored healthier sleep and blunted tau-driven brain damage in Alzheimer’s-model mice, pointing to sleep circuits as a lever against neurodegeneration. By blocking orexin signaling, the drug reduced abnormal tau and inflammation, an approach that could complement anti-amyloid therapies. Credit: Shutterstock
Lead author Samira Parhizkar, PhD, and senior author David M. Holtzman, MD, show that targeting sleep-regulating circuits can influence molecular pathways linked to Alzheimer’s disease and other tauopathies. The findings suggest orexin receptor antagonists such as lemborexant may be useful adjuncts to existing anti-amyloid therapies, which to date have had only partial effects on disease progression.
Mechanism, experimental design, and results
Lemborexant is an orexin receptor dual antagonist; it blocks both orexin receptor types 1 and 2. Orexins are neuropeptides that promote wakefulness and modulate physiological processes including sleep-wake cycles and appetite. By inhibiting orexin signaling, lemborexant altered sleep architecture in treated mice and appeared to reduce abnormal phosphorylation and aggregation of tau protein. Excess phosphate tags on tau drive its clumping, which triggers inflammation and neuronal death in several neurodegenerative diseases.
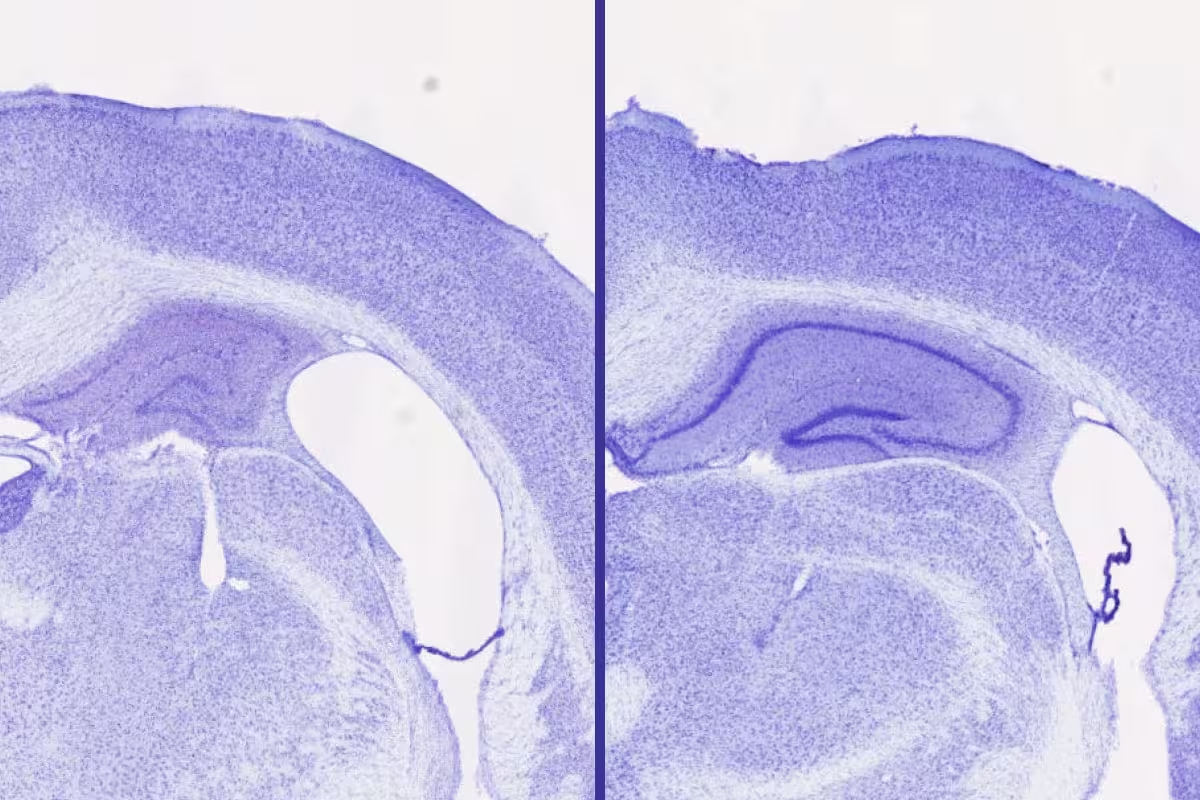
A new study by WashU Medicine researchers suggests that lemborexant and sleep aids that work the same way could help treat or prevent damage caused by harmful buildup of the protein tau in multiple neurodegenerative diseases, including Alzheimer’s. Shown are cross-sections of brain tissue from two mice genetically prone to tau accumulation. Treatment with lemborexant (right) results in larger volume in the hippocampus (central purple spiral), important for memory, and a smaller gap in brain tissue (white space) compared with no treatment (left). Credit: Samira Parhizkar/WashU Medicine
In genetically engineered mice that normally develop tau pathology, lemborexant-treated males retained 30 to 40 percent greater hippocampal volume than untreated controls. The study included a direct comparison with zolpidem, a sedative-hypnotic that enhances GABA signaling: zolpidem increased sleep but did not reduce tau accumulation or associated neurodegeneration. This contrast supports the idea that the neuroprotective effect depends on orexin-pathway modulation rather than sleep time alone. Importantly, lemborexant did not impair motor coordination in these models, a key consideration for potential use in people with cognitive impairment.
The researchers observed the beneficial effects primarily in male mice, a sex-specific outcome they are investigating further. Holtzman notes that female mice in this model developed less severe degeneration at baseline, which may have limited the detectable benefit of the drug in that group.
Implications for Alzheimer’s care and future research
This preclinical work identifies sleep circuits as a modifiable contributor to tau pathology. Because current FDA-approved treatments that target amyloid reduce some aspects of disease but do not fully stop progression, combining anti-amyloid therapies with agents that curb tau accumulation could be a promising strategy. Lemborexant and other orexin receptor antagonists already have safety and pharmacology data from human use as insomnia treatments, potentially accelerating translational studies.
However, mouse results do not guarantee human benefit. Clinical trials will be required to test whether orexin antagonists slow tau accumulation, reduce inflammation, and preserve cognition in people at risk for or in early stages of Alzheimer’s and related tauopathies. The drug studied was provided by Eisai as part of a collaborative research effort.
Related technologies and research directions
Future work may explore combination approaches that target both amyloid and tau, biomarkers to monitor tau phosphorylation noninvasively, and whether chronotherapy or optimized dosing schedules improve outcomes. Understanding sex differences, long-term safety in older adults, and interactions with other neurodegenerative treatments will be essential.
Expert Insight
Dr. Elena Morales, clinical neurologist and sleep researcher, comments: 'These results are compelling because they point to a mechanism-based approach rather than symptomatic sleep promotion alone. Leveraging drugs that reshape sleep circuitry could open a new therapeutic axis against tau. But translating mouse findings to human trials will require careful biomarker-driven designs and attention to sex-specific effects.'
Conclusion
This study positions lemborexant and similar orexin receptor antagonists as promising candidates to modify tau pathology through sleep-circuit modulation. While preclinical findings support a potential role in preventing or slowing tau-related neurodegeneration, clinical trials are needed to evaluate efficacy, optimal dosing, and safety in people with or at risk for Alzheimer’s disease and other tauopathies.
Source: scitechdaily


Leave a Comment