6 Minutes
A new laboratory study from Aarhus University points to a mechanism by which alpha‑synuclein oligomers — small assemblies of a protein already implicated in Parkinson’s disease — can physically breach cell membranes and create transient pores. These microscopic openings permit uncontrolled passage of ions and small molecules, potentially disturbing cellular chemistry and contributing to neuronal dysfunction. The findings offer a more detailed picture of how protein aggregates may harm brain cells and suggest new targets for therapeutic intervention.
Scientific background: alpha‑synuclein, oligomers and neurodegeneration
Alpha‑synuclein is a normal neuronal protein involved in synaptic vesicle trafficking and neurotransmitter release. In Parkinson’s disease it can misfold and accumulate into larger fibrils known as Lewy bodies. Alongside these fibrils, smaller soluble assemblies called oligomers are commonly observed — and many researchers consider oligomers to be particularly toxic. The new study focuses on those oligomers and their physical interaction with lipid bilayers, the basic structural element of cell membranes.
Oligomer‑induced membrane disruption has been proposed before, but the Aarhus team used a simplified, well‑controlled membrane model to visualize the process in unprecedented detail. By isolating this interaction they were able to record stepwise changes that lead from contact to pore formation.
Experimental setup and key observations
Using synthetic membrane models that mimic neuronal lipid composition, researchers introduced alpha‑synuclein oligomers while monitoring membrane integrity with high‑resolution imaging and electrical measurements. The team describes a three‑stage process: initial surface attachment of oligomers to the membrane, partial insertion into the lipid bilayer, and final reorganization into a pore structure that spans the membrane.
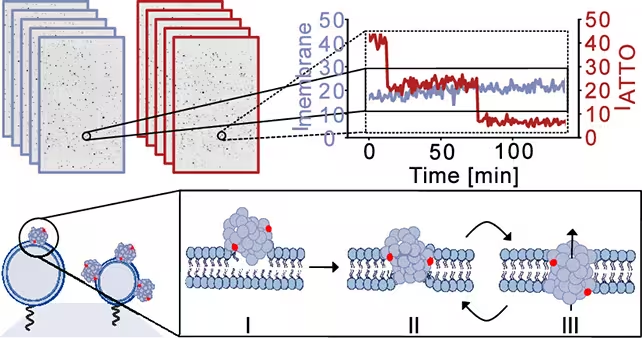
The researchers took snapshots of the simulated cell membranes to monitor how they were breached and the leakage caused. (Bro̷chner et al., ACS Nano, 2025)
Once formed, the pores were not static holes. Instead, they displayed dynamic behavior — repeatedly opening and closing on short timescales. That intermittency is important because it can cause episodic ion fluxes that gradually perturb cellular homeostasis rather than causing immediate catastrophic failure.
Membrane curvature preference and mitochondrial vulnerability
A notable observation was the oligomers’ preference for more curved membranes. Highly curved membranes are characteristic of intracellular organelles such as mitochondria. Mitochondria are central to neuronal energy production and calcium buffering; if their membranes are repeatedly punctured, it could trigger energetic failure, oxidative stress, and downstream pathways linked to neurodegeneration.
Implications for Parkinson’s disease mechanisms and therapy
If alpha‑synuclein oligomers form transient pores in neuronal membranes in vivo, this would provide a direct mechanism for how protein assemblies can cause functional decline before cell death. Transient ionic imbalances, repeated over time, can impair synaptic signaling, disrupt calcium homeostasis, and gradually degrade neuronal resilience. The dynamic, reversible nature of the pores may also explain why neurons often remain viable for long periods in Parkinson’s disease despite progressive dysfunction.
Molecular biologist Bo Volf Brøchner, a coauthor on the study, suggests that the opening and closing of pores could let cellular pumps temporarily compensate, delaying rapid collapse. That compensatory window may represent an opportunity for therapeutics to restore balance or block pore formation.
The Aarhus team has already explored molecular probes called nanobodies — small antibody fragments — that can recognize oligomeric alpha‑synuclein after assembly. While these nanobodies are useful for detection, they have not yet succeeded in preventing pore formation. Future therapeutic strategies might aim to:
- Stabilize alpha‑synuclein in non‑toxic conformations
- Directly block pore formation with small molecules or antibodies
- Protect or repair mitochondrial membranes
- Enhance cellular clearance of oligomers through proteostasis and autophagy pathways
Limitations and next steps
The experiments used clean, synthetic systems to isolate single variables. That experimental clarity is a strength — but the findings now require validation in live neurons and animal models where membrane composition, protein partners, and cellular defenses are more complex. Researchers will need to confirm whether oligomer‑driven pore formation occurs in the intact brain, how frequently it happens, and which neuronal subtypes are most vulnerable.
Further work should also quantify the size, lifetime and ionic selectivity of the pores, and assess how they interact with cellular repair mechanisms. If confirmed in vivo, these pores could become a measurable biomarker for early neuronal stress and a target for disease‑modifying therapies.
Expert Insight
Commentary from a practising neuroscientist
Dr. Elena Marquez, Senior Lecturer in Neurobiology (fictional), comments: "This study provides a compelling mechanistic link between oligomeric alpha‑synuclein and membrane dysfunction. The stepwise visualization of attachment, insertion and pore formation helps reconcile biochemical toxicity with physiological outcomes — episodic ion dysregulation rather than immediate necrosis. From a translational perspective, the challenge now is to demonstrate these events in intact neurons and to design molecules that either prevent pore assembly or rapidly seal transient breaches. Targeting mitochondrial membrane integrity in parallel could be particularly valuable given the oligomers’ curvature preference."
Related technologies and future prospects
Several complementary technologies could accelerate translation of these findings into therapy or diagnostics:
- Advanced live‑cell imaging to capture transient pore events in neurons
- Biosensors for ionic fluxes and mitochondrial membrane potential
- High‑throughput screens for small molecules that block pore assembly
- Refinement of nanobody platforms to neutralize oligomers before membrane engagement
Altogether, these approaches could yield early‑stage interventions that slow progression by preventing repeated sublethal membrane injury.
Conclusion
The Aarhus University study advances our understanding of Parkinson’s disease by showing that alpha‑synuclein oligomers can form dynamic pores in model membranes through an attachment–insertion–pore formation sequence. These transient breaches, with a noted preference for curved membranes like mitochondrial surfaces, could produce episodic ionic disturbances that accumulate into neuronal dysfunction. While further validation in living systems is required, the results highlight novel mechanistic targets — from oligomer neutralization to membrane protection — that may guide future therapeutic and diagnostic development. The research, published in ACS Nano, opens a new avenue for exploring how small protein assemblies contribute to neurodegeneration and how that process might be interrupted before irreversible loss of function.
Source: sciencealert

Leave a Comment