8 Minutes
Researchers reveal an alternative heat-producing system in brown fat
Researchers at Washington University School of Medicine in St. Louis have identified a previously unrecognized cellular route by which brown adipose tissue (brown fat) burns fuel and produces heat. The discovery, reported in Nature, highlights a peroxisome-centered process that can act as a "backup heater" when the canonical mitochondrial thermogenic machinery is compromised. The pathway depends on the enzyme acyl-CoA oxidase 2 (ACOX2) and appears to influence whole-body metabolism, insulin sensitivity, and weight regulation in mice.
Brown fat differs from white adipose tissue (white fat) and skeletal muscle because its primary function is adaptive thermogenesis — converting calories into heat to maintain body temperature in cold environments. For decades, mitochondrial uncoupling protein 1 (UCP1) has been seen as the principal molecular switch that diverts mitochondrial energy generation into heat. Still, prior observations showed that animals lacking UCP1 can retain some capacity to generate heat, implying alternative thermogenic mechanisms.
Peroxisomes and ACOX2: a complementary thermogenic route
The new study puts peroxisomes — small organelles specialized in fatty acid metabolism — at the center of this alternative thermogenic system. Investigators found that exposure to cold stimulates peroxisome proliferation in brown fat. This response was even more prominent in mice genetically lacking UCP1, suggesting peroxisomes can compensate when mitochondrial uncoupling is impaired.
Lodhi and colleagues traced the compensatory activity to an enzyme in peroxisomes, acyl-CoA oxidase 2 (ACOX2). In mouse models where ACOX2 was removed specifically from brown fat, animals struggled to maintain normal body temperature during cold challenge and showed impaired insulin responsiveness. These mice gained more weight on a calorie-rich high-fat diet than their control counterparts.
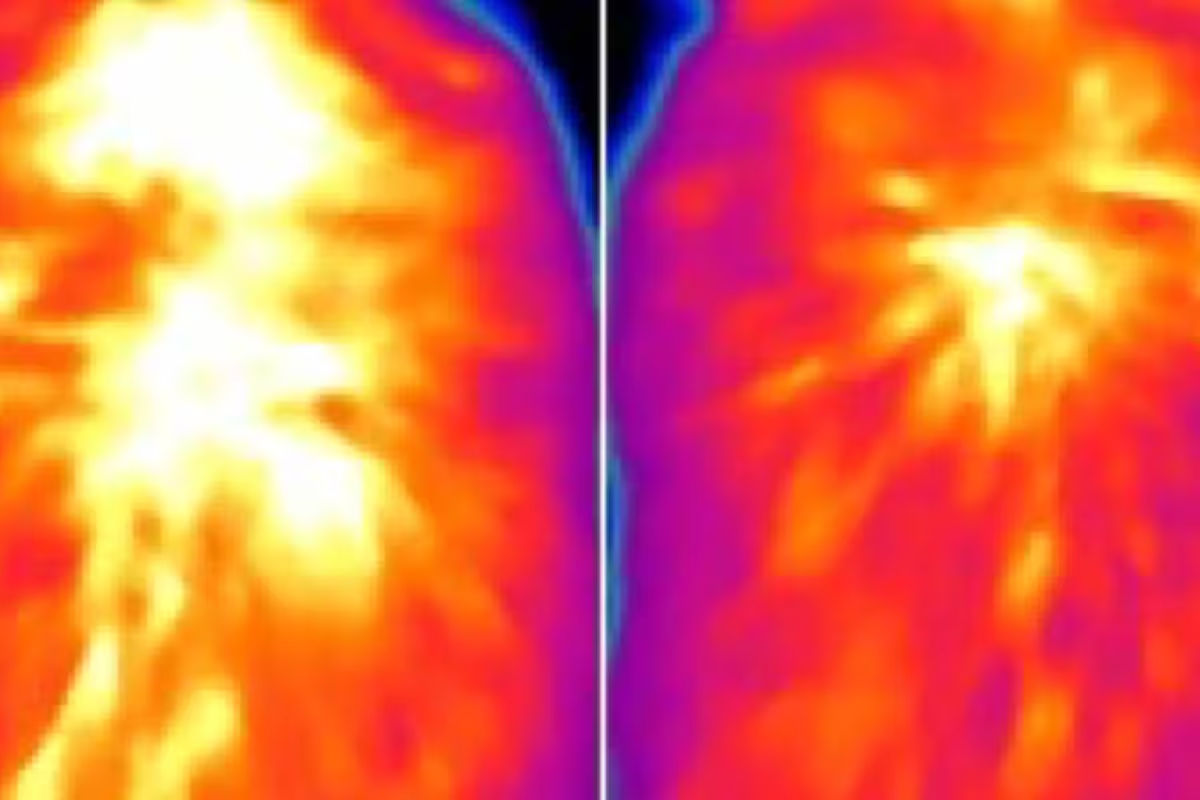
A new study led by researchers at WashU Medicine reveals possible new avenues to exploit brown fat to treat metabolic diseases, such as obesity. Infrared images indicate reduced levels of heat production from brown fat in a mouse lacking a protein called ACOX2 (right), compared to a control mouse. Restoring high levels of ACOX2 in brown fat led to increased heat production and better weight control even in mice on a high-fat diet.
Conversely, mice engineered to overexpress ACOX2 selectively in brown fat displayed enhanced thermogenesis, better cold tolerance and improved metabolic markers, including greater insulin sensitivity and protection from diet-induced obesity. Using a fluorescent heat sensor developed for the study, researchers directly observed that ACOX2-dependent metabolism of particular fatty acids increased intracellular heat in brown adipocytes. Complementary infrared thermal imaging confirmed reduced brown fat heat output in ACOX2-deficient animals.
Scientific background: how peroxisomal thermogenesis differs from mitochondrial pathways
Mitochondrial thermogenesis via UCP1 works by allowing protons to cross the inner mitochondrial membrane without driving ATP synthesis, releasing stored electrochemical energy as heat. Peroxisomal thermogenesis is mechanistically distinct: peroxisomes oxidize specific fatty acids in reactions that generate heat and metabolic intermediates without directly coupling to mitochondrial proton gradients. ACOX2 catalyzes the first step in the peroxisomal beta-oxidation of certain branched and long-chain fatty acids.
This alternative route effectively "wastes" chemical energy as heat, increasing resting energy expenditure. From a therapeutic perspective, enhancing peroxisomal activity in brown fat could raise daily caloric burn without requiring greater physical activity — a potential complement to dietary and exercise interventions.
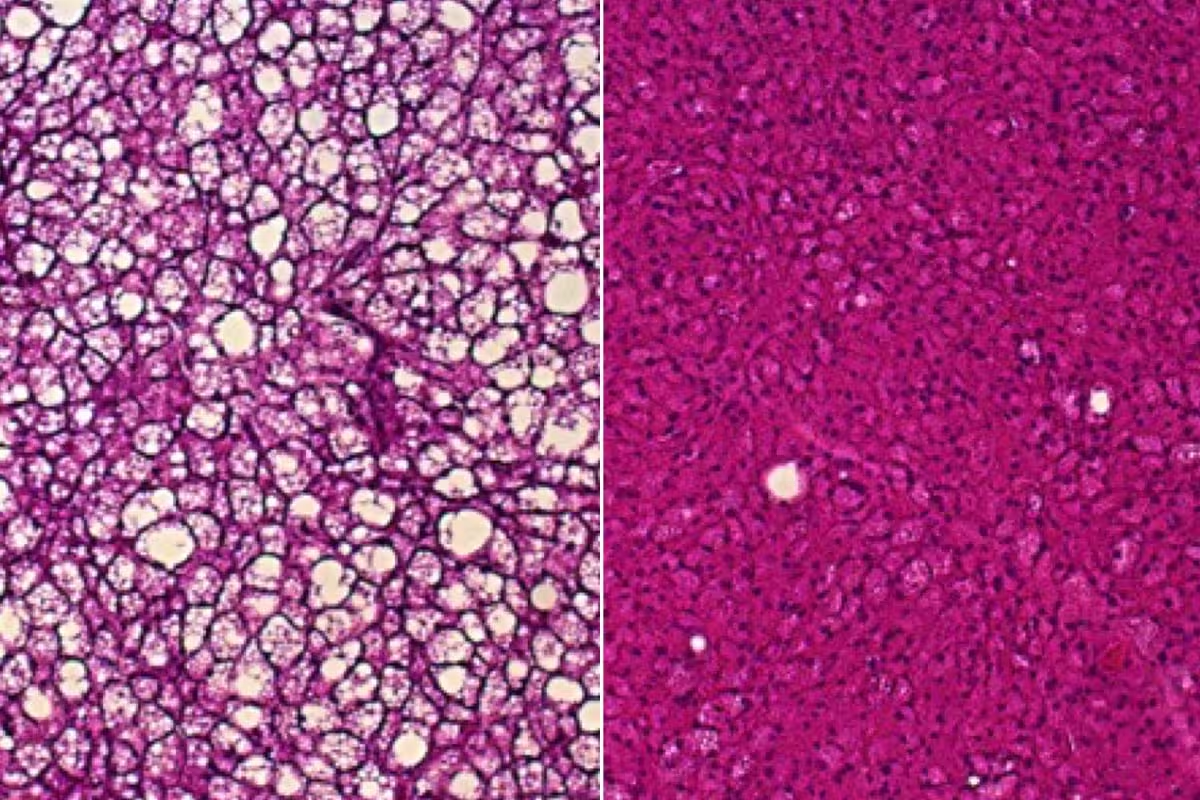
A new study led by researchers at WashU Medicine reveals possible new avenues to exploit brown fat to treat metabolic diseases, such as obesity. Brown fat tissue from mice deficient in uncoupling protein 1 and fed a high-fat diet accumulates white fat droplets (left), a sign of impaired heat production. In brown fat from mice also deficient in uncoupling protein 1 but genetically engineered to have unusually high amounts of the protein ACOX2, white fat doesn’t build up on a high-fat diet (right) and heat production is better.
Implications for human health and potential interventions
Although the current experiments were conducted in mice, several lines of evidence suggest this pathway could be relevant to humans. Certain fatty acids metabolized by ACOX2 are present naturally in human milk, dairy products and are produced by some gut microbes. Prior observational studies have linked higher circulating levels of these fatty acids to lower body mass index in humans, although causality remains unproven.
The authors of the study propose multiple translational approaches: dietary strategies to increase intake or endogenous generation of the relevant fatty acids (via prebiotics, probiotics or targeted food components), nutraceutical formulations, or small-molecule drugs that directly activate ACOX2 or promote peroxisome biogenesis in brown adipocytes. Each strategy would need rigorous preclinical safety and efficacy evaluation before human trials.
Senior author Irfan Lodhi, PhD, emphasized the therapeutic rationale: increasing peroxisome-driven thermogenesis could boost resting energy expenditure and help maintain weight loss. He noted the approach might be easier for some people to sustain than long-term calorie restriction or intense exercise, by augmenting basal metabolic processes rather than relying solely on behavioral change.
Experimental tools and technologies used
Key techniques in the study included:
- Mouse genetic models with tissue-specific deletion or overexpression of ACOX2 and UCP1.
- Cold-exposure protocols to trigger thermogenic responses.
- A fluorescent intracellular heat sensor designed to detect small, localized temperature changes inside brown adipocytes.
- Infrared thermal imaging to map heat output from brown fat depots in live animals.
- Metabolic phenotyping, including glucose and insulin tolerance tests, and body-composition analysis under high-fat diet conditions.
These methods enabled the team to connect molecular changes in peroxisomal function to measurable effects on whole-animal energy balance and glucose metabolism.
Expert Insight
"This research adds an important layer to our understanding of adipose tissue plasticity," says Dr. Elena Morales, a metabolic physiologist unaffiliated with the study. "Peroxisomes are often overlooked in energy-balance discussions, yet they can reshape cellular fuel use. If we can learn to safely modulate peroxisomal activity in human brown fat, it may offer a new avenue to support metabolic health without the systemic side effects that have limited some prior pharmacological approaches."
Future directions and challenges
Translating this discovery to human benefits raises several questions: Do human brown fat depots express ACOX2 at functionally meaningful levels? Which specific dietary fatty acids or microbial metabolites most effectively engage the pathway? Can peroxisome-targeted therapies be made tissue-specific to avoid off-target effects on liver or other organs where peroxisomal metabolism is critical?
Addressing these questions will require comparative studies in human tissue, carefully controlled dietary experiments, and medicinal chemistry to identify safe activators of ACOX2 or peroxisome biogenesis. Long-term safety will be especially important because altering basal energy expenditure could have complex endocrine and metabolic consequences.
Conclusion
The discovery of ACOX2-dependent peroxisomal thermogenesis in brown fat uncovers a novel mechanism by which organisms can expend energy and maintain metabolic health. In mice, enhancing this pathway improved cold tolerance, insulin sensitivity and resistance to diet-induced obesity; loss of the pathway produced the opposite effects. While substantial work remains to assess translational potential in humans, the findings expand the toolbox of possible interventions for obesity and metabolic disease, pointing to diet-derived fatty acids, gut microbiota modulation, and direct pharmacologic activation of peroxisomal pathways as promising avenues for future research.
Source: scitechdaily


Leave a Comment