6 Minutes
Stem cell transplantation reverses stroke damage in mice
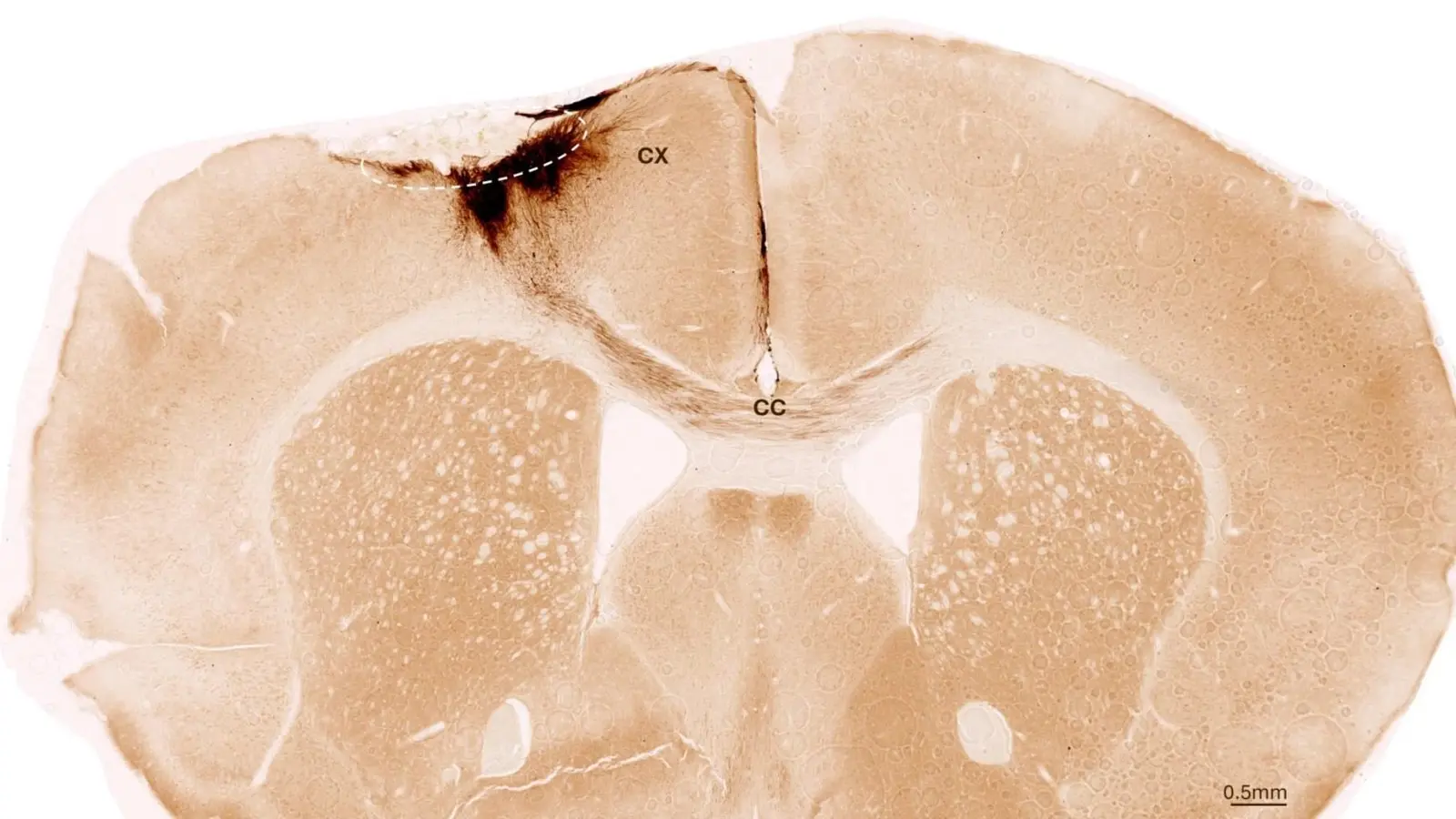
This image shows a coronal section through the mouse brain after stroke and neural stem cell transplantation. The dashed circle indicates the stroke area. The neurite projections of the transplanted human cells are stained in dark brown. Neurites extend locally into the cortex (CX) but also via the corpus callosum (CC) into the other brain hemisphere. Credit: University of Zurich
Researchers at the University of Zurich (UZH), in collaboration with teams in the United States and Japan, have demonstrated that human neural stem cells can regenerate damaged brain tissue after stroke in mice. The transplanted cells survived for weeks, differentiated into neurons that integrated with host circuits, and triggered broader repair processes that improved motor function. These results mark an important step toward clinical strategies for brain regeneration and recovery after stroke.
Scientific context and experimental design
Stroke is a leading cause of adult disability worldwide; approximately one in four adults will experience a stroke in their lifetime. Ischemic stroke and intracerebral hemorrhage both kill neurons and other brain cells, often irreversibly, producing long-term impairments in movement, speech, and cognition. Conventional treatments focus on limiting acute damage and rehabilitation; today, no established therapy can replace lost neurons or fully rebuild damaged brain tissue.
To test whether neural stem cells can reconstruct injured brain tissue, the research teams used human neural stem cells derived from induced pluripotent stem cells (iPSCs). iPSCs originate from normal somatic (adult) human cells that have been reprogrammed to a pluripotent state, then differentiated into neural stem cells capable of forming multiple neural cell types.
The investigators induced a permanent focal stroke in mice that reproduces key pathological and behavioral features of human stroke. The animals were immunologically modified to avoid rejection of human cells, enabling long-term observation of cell fate. One week after stroke, the team transplanted neural stem cells into the lesion site — a timing chosen deliberately to mimic a clinically realistic window and to allow the local environment to stabilize.
For outcome measures the group used multimodal approaches: histology and immunostaining to track cell survival and differentiation, molecular assays to assess inflammation and vascular integrity, and high-resolution imaging to evaluate structural integration. Behavioral recovery was quantified using automated, AI-assisted gait analysis to detect improvements in motor coordination and limb use.
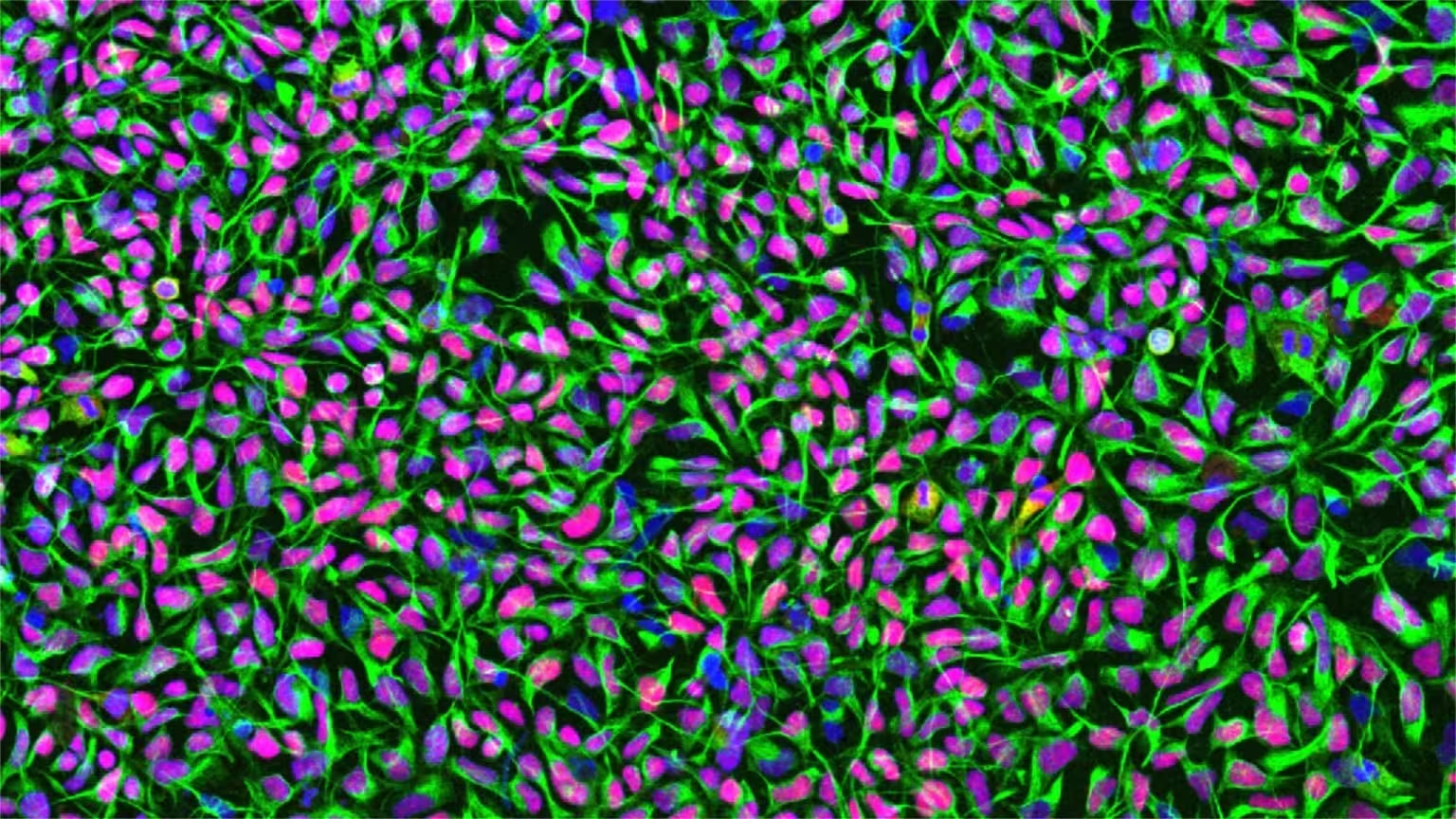
Human neural stem cells in culture. Cell nuclei are stained in blue, the neural stem cell-specific filament protein Nestin is shown in green, and the neural stem cell transcription factor Sox1 in red. Credit: University of Zurich
Major findings: neurogenesis plus systemic repair
The transplanted human neural stem cells survived throughout the five-week observation period and predominantly matured into neurons. Importantly, many of these newly generated neurons extended neurites and formed putative synaptic contacts with the host circuitry, indicating functional integration rather than transient presence.
Beyond direct neurogenesis, the researchers observed several ancillary regenerative effects:
- Angiogenesis and vascular repair: New blood vessel formation increased in and around the lesion, supporting metabolic recovery.
- Reduced inflammation: Local inflammatory responses—known to perpetuate secondary damage—were attenuated following transplantation.
- Improved blood-brain barrier (BBB) integrity: Measures of BBB function indicated better containment of the brain microenvironment, limiting harmful infiltration.
Together these processes created a more permissive milieu for tissue regeneration and functional restoration. Behavioral assays confirmed partial reversal of stroke-induced motor deficits; mice receiving stem cell grafts showed measurable improvements in gait and limb coordination compared with controls, verified by AI-assisted analysis.
Translation considerations and safety strategies
Recognizing the translational goal, the researchers produced stem cells using xeno-free protocols (i.e., without animal-derived reagents), developed in collaboration with the Center for iPS Cell Research and Application (CiRA) at Kyoto University. Xeno-free manufacturing is an important regulatory and safety requirement for human clinical use.
Timing emerged as a clinically relevant variable: the studies found better outcomes when transplantation was performed one week after stroke rather than immediately. This delayed window may simplify logistics for cell production and patient selection in future trials.
Safety remains a central challenge. The investigators are developing engineered ‘‘safety switch’’ systems to eliminate uncontrolled cell proliferation, and they are exploring less invasive delivery routes such as endovascular injection, which would be more feasible than direct parenchymal grafting in many clinical scenarios. Parallel clinical programs using iPSC-derived cells in Parkinson’s disease are already underway in Japan, showing a pathway for regulatory approval and early human testing. Stroke is a plausible next indication for carefully designed trials.
Expert Insight
"These results provide compelling evidence that neural stem cells can do more than replace lost neurons — they reshape the local environment to promote vascular and immune recovery as well," says Dr. Laura Hernandez, a fictional neurorehabilitation specialist and translational neuroscientist. "Translating this approach to patients will require rigorous safety measures, scalable manufacturing under clinical-grade conditions, and well-defined delivery methods. But the prospect of restoring function after stroke is now closer than it was five years ago."
Conclusion
The UZH-led studies demonstrate that human neural stem cells derived from iPSCs can survive, differentiate, and meaningfully contribute to structural and functional recovery after experimental stroke in mice. By combining neurogenesis with vascular repair and immunomodulation, stem cell transplantation produced measurable motor improvements. Ongoing work on safety switches, xeno-free manufacturing, and minimally invasive delivery will determine how rapidly these findings can be advanced into clinical trials. If successfully translated, neural stem cell therapy may offer a transformative option for restoring brain function after stroke.
Source: scitechdaily

Leave a Comment