4 Minutes
Researchers have identified a common genetic architecture that connects eight major psychiatric disorders, offering new insight into why these conditions frequently co-occur and cluster in families. A study published in Cell this year mapped specific gene variants that act across multiple stages of brain development and across diverse brain cell types, revealing mechanisms that could be targeted by broad-spectrum therapies.
The disorders examined include autism spectrum disorder, attention-deficit/hyperactivity disorder (ADHD), schizophrenia, bipolar disorder, major depressive disorder, Tourette syndrome, obsessive-compulsive disorder (OCD), and anorexia nervosa. Prior large-scale analyses first identified 109 genes associated— in different combinations— with these eight conditions. The new study drills down to particular genetic variants and tests how they influence gene regulation in developing neural cells.
Background and study design
Why this matters
Many psychiatric diagnoses overlap clinically: for example, up to 70% of people with autism or ADHD also meet diagnostic criteria for the other condition at some point. Shared genetic causes help explain co-occurrence and familial clustering. The new work focuses on pleiotropy—where a single genetic variant influences multiple traits or disorders—and examines how pleiotropic variants operate during neurodevelopment.
Experimental approach
The research team compiled nearly 18,000 genetic variants drawn from both disorder-specific and shared gene sets. They introduced these variants into human neural precursor cells—the progenitors that differentiate into brain neurons—and measured the impact on gene expression and regulatory networks during simulated brain development. The authors validated key regulatory variants in developing mouse neurons to confirm functional effects across species.
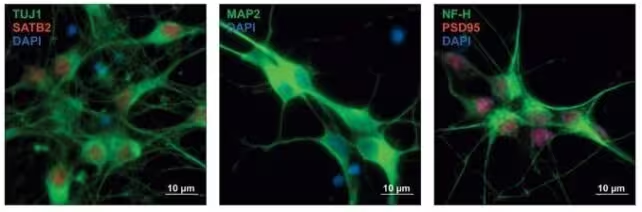
Human precursor neurons with protein expression stained in different colors, indicating the type of neurons developing. (Won et al., Cell, 2025)
Key findings
The study identified 683 variants that alter gene regulation in neural precursor cells. Variants classified as pleiotropic showed several distinguishing features:
- Greater connectivity: Pleiotropic variants affect proteins that participate in many more protein–protein interactions than disorder-specific variants. This means a change in one protein can ripple through interaction networks and influence wide aspects of brain function.
- Broader cell-type activity: These variants remain active in multiple brain cell types and across extended developmental windows, increasing the chance they will affect successive stages of circuit formation.
- Regulatory cascade effects: Pleiotropic variants modulate gene-regulatory mechanisms across developmental stages, explaining how the same variant can contribute to different clinical outcomes depending on timing and cell context.
"The proteins produced by these genes are also highly connected to other proteins," said University of North Carolina geneticist Hyejung Won. "Changes to these proteins in particular could ripple through the network, potentially causing widespread effects on the brain." This network-centered view positions pleiotropic variants as high-leverage nodes for both risk and intervention.
Implications for diagnosis and treatment
Understanding shared genetic drivers could shift how clinicians and researchers approach psychiatric classification and therapy development. Historically, pleiotropy complicated disorder taxonomy; now it offers a potential advantage: targeting shared molecular pathways could yield treatments effective across multiple conditions. Because pleiotropic variants influence broad interaction networks and developmental trajectories, interventions aimed at stabilizing those networks may reduce risk or symptoms for several disorders simultaneously.
The World Health Organization estimates about 1 in 8 people live with a psychiatric condition globally—nearly one billion individuals—so therapies that address shared causes could have large public-health impact.
Conclusion
This study advances the genetic and developmental map linking diverse psychiatric disorders through pleiotropic variants that operate across cell types and developmental stages. By clarifying which variants alter regulatory networks, the research offers tangible targets for future functional studies and for therapeutic strategies designed to modulate shared pathways underlying multiple psychiatric conditions.
Source: sciencealert

Leave a Comment