5 Minutes
A compact, low-cost chemical has shown striking effects in an experimental rat model of Alzheimer's disease, reversing memory deficits and reducing brain inflammation. The discovery zeroes in on a surprising culprit: excess copper trapped in beta-amyloid plaques. Scientists behind the study say the molecule is safe in preclinical tests and could move into human trials—raising cautious hope for a new therapeutic avenue.
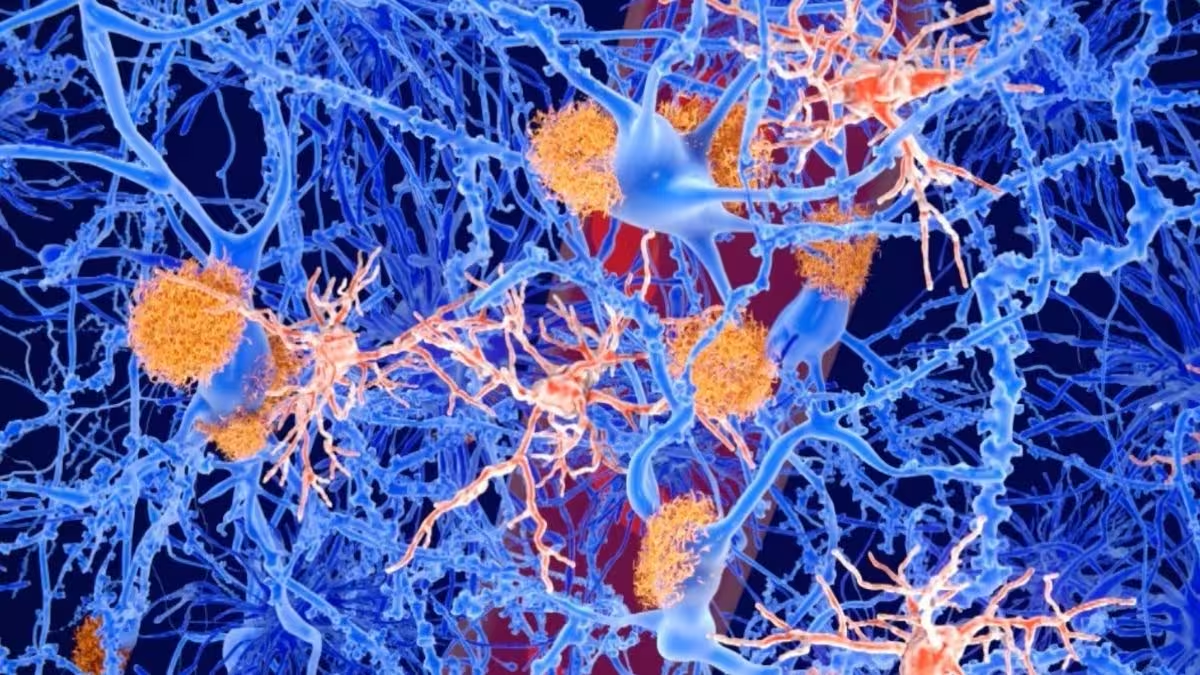
Artist's impression of amyloid plaques (orange) accumulating on neurons (blue)
Why copper matters in Alzheimer's research
Alzheimer's disease is commonly associated with clumps of beta-amyloid proteins in the brain. Whether those plaques are the cause of cognitive decline or a downstream effect remains debated, but they are a persistent target for drug development. One biochemical thread that has gained attention over the past decade is metal ion dysregulation—particularly copper.
Copper is an essential trace metal required for normal brain function. However, when copper homeostasis goes awry—because of genetic changes or altered copper-transport enzymes—the metal can accumulate around beta-amyloid deposits. That excess copper can accelerate plaque aggregation and foster oxidative stress, a form of cellular damage implicated in neuron loss and cognitive decline.
From computer screens to rat mazes: how the team tested compounds
Researchers at the Federal University of the ABC in Brazil screened a suite of nine candidate molecules designed to remove, or chelate, excess copper from beta-amyloid. Eight were imines (organic molecules with a carbon-nitrogen double bond) and one was quinoline-based. Initial in silico simulations identified three promising candidates—labelled L09, L10 and L11—that appeared capable of crossing the blood-brain barrier, an essential property for any drug intended to act in the brain.
Next came cell-based toxicity tests. Mouse neurons grown in culture were exposed to the three shortlisted compounds for 24 hours. One candidate, L11, increased oxidative stress and damaged cells—effectively disqualifying it. The two imine compounds, L09 and L10, showed low toxicity and protected lipids and DNA from oxidative damage, marking them as safer options for animal testing.
The team then moved into an established rat model of Alzheimer's. Researchers induced Alzheimer-like pathology by injecting streptozotocin to disrupt insulin signaling in the brain, a method that promotes beta-amyloid accumulation and cognitive impairment. Treated animals were assessed for copper levels, markers of inflammation and oxidative stress, and performance in spatial memory tests such as mazes.
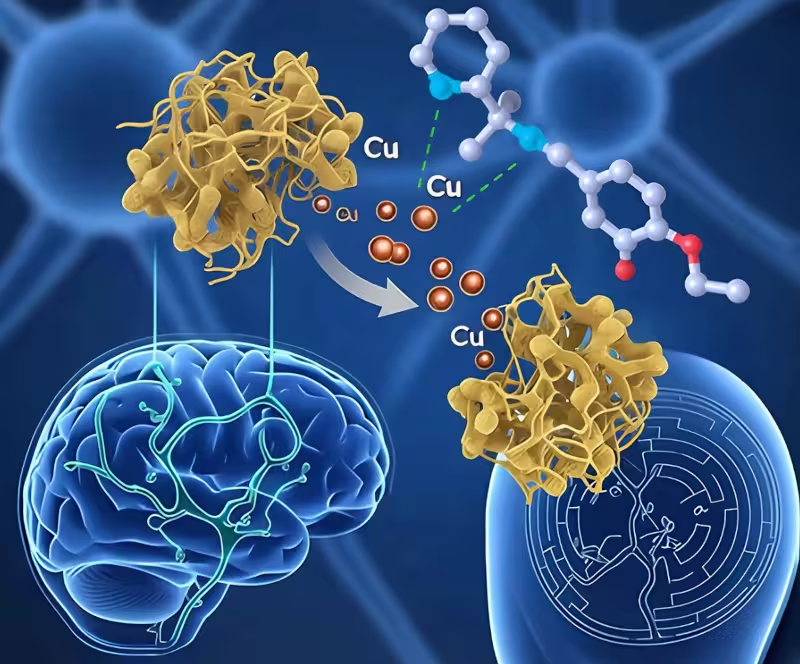
The new compound can strip beta-amyloid clumps of excess copper
Key discovery: L10 restores copper balance and memory
Of the three candidates, compound L10 emerged as the clear leader. Rats treated with L10 showed normalized copper concentrations in the hippocampus—the brain region most closely tied to short- and long-term memory. The compound also cut levels of neuroinflammation and oxidative stress, and treated rats navigated mazes far better than untreated controls.
L09 produced weaker effects across the same measures, and L11 remained harmful in cellular assays. Based on these results, the authors identified L10 as the most promising candidate for further development and potential human testing.
Lead investigator Giselle Cerchiaro (UFABC) emphasized the practical advantages of the molecule: it is chemically simple and inexpensive to produce compared with many current experimental drugs. That affordability could be important if the compound proves effective in patients, since Alzheimer's is a global epidemic affecting an estimated 55 million people.
What this means for Alzheimer's treatment strategy
Most approved Alzheimer's therapies today offer symptomatic relief but do not reverse underlying pathology. A strategy that restores metal balance in the brain—reducing copper-driven plaque aggregation and oxidative damage—represents a different therapeutic angle. Importantly, the study suggests such an approach might benefit a subgroup of patients with copper dysregulation rather than everyone with Alzheimer's, highlighting the disease's biological heterogeneity.
Expert Insight
"Targeting metal homeostasis is a pragmatic and mechanistic strategy," says Dr. Anna Morales, a neurologist and translational researcher not involved in the study. "This work is encouraging because it combines a clear biochemical hypothesis with stepwise preclinical testing—molecular modeling, cell assays, and animal behavior. The next big hurdles will be safety and efficacy in humans, and whether the compound helps the right patient subgroups."
Next steps and clinical prospects
The researchers aim to progress L10 into human trials to determine safety, tolerability and potential cognitive benefits in people. Questions remain: How well will the compound perform across diverse patient populations? Can it be combined with other therapies? And how long-lasting are the cognitive improvements? Answering these will require carefully designed clinical studies guided by biomarkers of copper imbalance and amyloid pathology.
For now, L10 represents an intriguing, low-cost candidate that shifts attention to metal chemistry in the brain—an area that could yield targeted therapies for a subset of Alzheimer's patients. As always, promising animal results must be validated in human trials before clinical use, but the study provides a clear template for moving from molecular insight to potential therapy.
Source: sciencealert
Comments
Reza
Is copper buildup really the main driver? Sounds promising, but how will they id patients with that subtype? quick thought.
neurobit
wow this is wild, cheap drug reversing memory in rats? if true could change everything… but human trials tho.

Leave a Comment