5 Minutes
Researchers at Baylor College of Medicine have uncovered a surprising, built-in mechanism the brain can use to attack Alzheimer’s pathology. By increasing levels of a regulator protein called Sox9, scientists switched on star-shaped support cells known as astrocytes and turned them into potent cleaners of amyloid plaques — the sticky protein deposits long linked to memory loss.
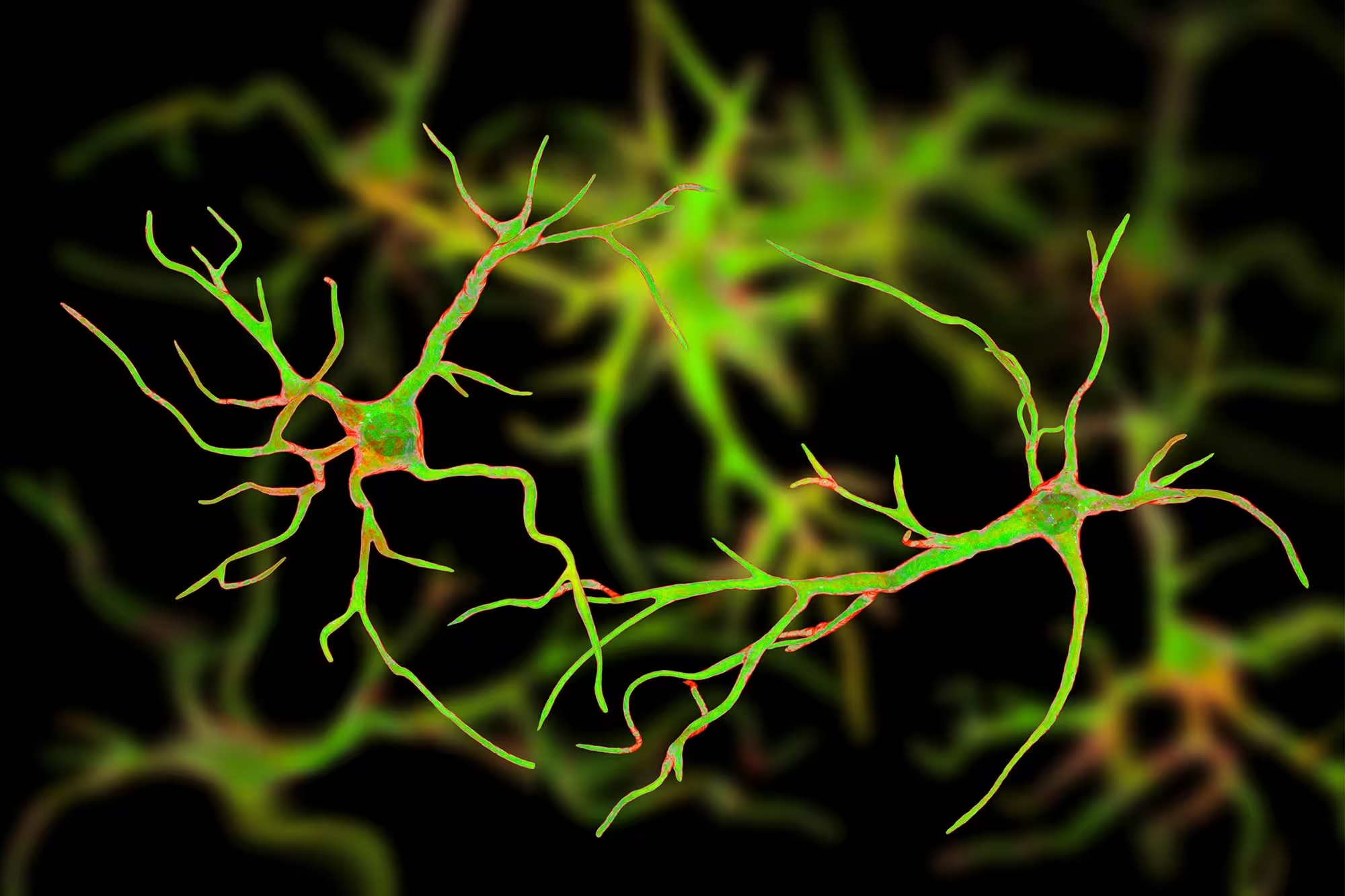
Boosting Sox9 activates astrocytes’ natural ability to clean up amyloid plaques and protect cognitive function. The results point toward an entirely new therapeutic angle for Alzheimer’s centered on the brain’s support cells, not neurons.
Astrocytes: the brain’s overlooked housekeepers
Astrocytes are glial cells that do far more than hold the brain together: they regulate neurotransmitter levels, support synapses, and help maintain the chemical environment neurons need to function. In aging and neurodegenerative disease, astrocytes change their behavior and gene expression, but until now their potential to actively remove existing amyloid plaques remained underappreciated.
What is Sox9 and why it matters
Sox9 is a transcription factor — a protein that controls the activity of many genes. In the aging brain, Sox9 appears to act as a master regulator for critical astrocyte functions. By modulating Sox9, researchers can tilt astrocytes toward a more active, complex state that enhances their ability to ingest and clear amyloid-beta deposits.
How the experiment worked: testing Sox9 in mice with symptoms
The Baylor team focused on Alzheimer’s models that already showed cognitive decline and amyloid plaque accumulation, which makes the work more relevant to human disease where pathology is often well established by the time symptoms appear. Rather than preventing plaque formation, the key question was whether existing plaques could be cleared and cognition preserved.
Using genetic tools, the investigators either increased Sox9 expression in astrocytes or removed it from these cells. They then tracked individual mice for six months, using behavioral tests that measured memory and recognition of familiar objects and environments. After the behavioral phase, brains were analyzed to quantify plaque burden and assess astrocyte morphology and activity.
Key discovery: Raising Sox9 reverses plaque build-up and memory loss
Results showed a clear pattern. Lowering Sox9 accelerated plaque accumulation, simplified astrocyte structure, and reduced the cells’ ability to clear amyloid. By contrast, overexpressing Sox9 made astrocytes more complex and active; these cells engulfed more amyloid, decreased plaque load, and — importantly — preserved cognitive performance in mice that had previously shown impairment.
In plain terms, boosting Sox9 converted astrocytes into a more efficient cleanup crew, reducing the pathological hallmark of Alzheimer’s and halting associated memory decline in animal models. This points to a therapeutic strategy that complements neuron-focused approaches by empowering the brain’s own support cells.
Therapeutic promise and necessary cautions
These findings open a new therapeutic avenue: instead of only trying to block plaque formation or protect neurons, treatments might one day stimulate astrocytes to clear existing amyloid deposits. Because astrocytes are abundant and play multiple roles in brain homeostasis, targeting them could have broad effects — both beneficial and potentially risky.
Researchers stress several caveats. The current data come from mice, and human brains differ in complexity and timescale. Long-term consequences of artificially boosting Sox9 are unknown; overstimulation of glial cells can sometimes trigger inflammation or impair other functions. More work is needed to map Sox9-dependent pathways, identify safe delivery methods, and verify effects in human tissue or clinical trials.
Related science and future directions
This study intersects with growing interest in glial biology, neuroimmunology, and gene-regulation therapies. Techniques such as viral vectors, small molecules that modulate transcription factors, or gene-editing tools could potentially be adapted to tweak Sox9 activity. Parallel efforts will need to address timing — whether early or later intervention provides the best benefit — and to ensure interventions do not disrupt the many essential roles astrocytes perform.
Expert Insight
"The work at Baylor highlights a shifting paradigm: support cells are not passive bystanders in neurodegeneration," says Dr. Emily Saunders, a neurobiologist at the Institute for Brain Health. "If we can safely harness astrocytes' innate clearance mechanisms, it could complement existing approaches and offer a route to reduce pathology even after symptoms appear. The challenge now is translating those gene-level changes into therapies that are effective and safe in people."
In short, the study adds an important piece to the Alzheimer’s puzzle. It suggests that the brain may already have tools to fight back — we just need to understand how to switch them on without causing harm. As researchers dissect Sox9’s downstream effects and test translation strategies, the concept of astrocyte-powered therapies moves from speculative to plausible.
For patients and caregivers, these results do not yet change clinical care. But they expand the map of therapeutic targets and reinforce the idea that successful Alzheimer’s treatments may require a multipronged approach: protect neurons, limit toxic protein spread, and activate the brain’s own cleanup systems.
Source: scitechdaily
Comments
Tomas
is this even true? mice had plaques cleared after Sox9 boost, but humans are WAY more complex. also long term astrocyte overdrive = inflammation? curious but skeptical
bioNix
wow didnt expect astrocytes to be switchable, Sox9 turning them into plaque cleaners? If that holds up in humans this could be huge but im cautious

Leave a Comment