5 Minutes
Background: KRAS mutations and unmet needs in pancreatic and colorectal cancer
Pancreatic and colorectal cancers are among the most treatment-resistant malignancies, with high rates of recurrence after surgery and conventional therapy. A driving factor in many cases is mutation of the KRAS oncogene, which produces abnormal signaling proteins that promote tumor growth. KRAS mutations are implicated in roughly 93% of pancreatic cancers and about 50% of colorectal cancers, making the gene a high-value target for therapeutic intervention. Immunotherapy vaccines that train the immune system to recognize and eliminate KRAS-mutant cells could reduce relapse and extend survival if they generate a durable anti-tumor T cell response.
Trial design and how ELI-002 2P works
ELI-002 2P is an "off-the-shelf" peptide vaccine designed to stimulate T cells against common mutant forms of the KRAS protein. Unlike bespoke personalized vaccines that must be manufactured to match a patient’s unique tumor mutations, an off-the-shelf product is standardized and ready for broader clinical use. The vaccine couplestargeted KRAS-derived peptides with a delivery system intended to ferry the antigen directly to lymph nodes, where naive and memory immune cells are concentrated. By focusing antigen presentation in lymphoid tissue, the platform aims to generate a focused and robust expansion of mutant-KRAS-specific T cells.
The initial clinical evaluation enrolled patients who had undergone surgery to remove primary tumors but showed evidence suggesting a high likelihood of relapse. Participants included 20 people treated after pancreatic cancer surgery and 5 after colorectal cancer resection. The protocol involved repeated vaccine injections and immunologic monitoring to measure T cell responses and clinical follow-up to document relapse-free survival and overall survival.
Key results: immune responses and clinical outcomes
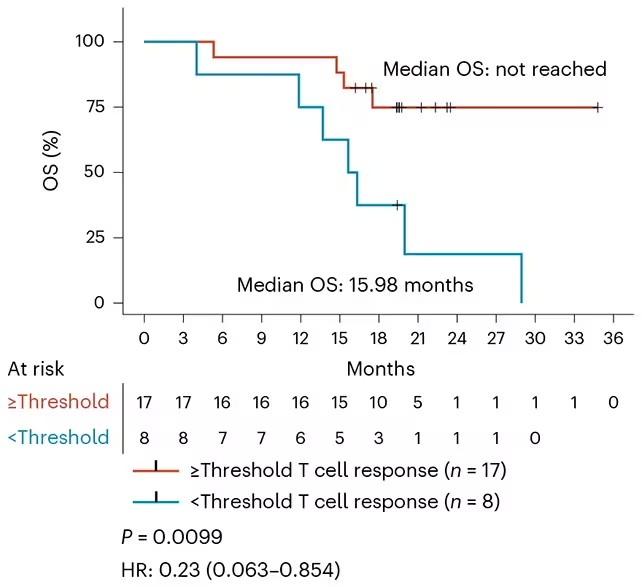
The trial produced encouraging signals. A large majority—84%—of participants developed T cells specifically reactive to mutant KRAS peptides, indicating effective priming of adaptive immunity. In 24% of participants, circulating or tissue-detectable traces of tumor-derived KRAS products became undetectable after vaccination. Among the 24 people who mounted the strongest immune responses, 17 remained disease-free at the last scheduled follow-up, with a median observation of nearly 20 months.
Across the cohort, median relapse-free survival was 16.33 months and median overall survival reached 28.94 months—outcomes that compare favorably with historical expectations for these aggressive cancers. Investigators also noted evidence that the immune responses induced by ELI-002 2P could broaden to target additional cancer-associated alterations, suggesting potential cross-reactivity that may increase clinical benefit in heterogeneous tumors.
Safety and tolerability
The early-phase study focused on safety and immunogenicity. No unexpected safety signals were evident in this small cohort, and the vaccine was generally well tolerated. Larger trials are required to fully characterize adverse events and long-term safety.
Implications, expert perspective and next steps
Medical oncologists involved in the study highlighted the importance of these findings for KRAS-driven tumors. As one investigator noted, producing a standardized vaccine that reliably induces KRAS-specific T cells could change the management of cancers that historically recur despite surgery and chemotherapy. Because ELI-002 2P is off-the-shelf, it avoids the manufacturing delays and costs associated with individualized vaccines, improving scalability for broader patient populations.
Further research is needed: randomized controlled trials with larger patient numbers will be essential to confirm survival benefits, define which patients derive the most benefit, and evaluate combinations with other immunotherapies or targeted agents. Additional studies will also characterize biomarkers that predict response and explore extending the platform to other oncogenic mutations.
Conclusion
The early clinical data for ELI-002 2P indicate that a lymph node-targeted, off-the-shelf vaccine can safely induce robust T cell responses against KRAS-mutant tumor cells and produce clinical outcomes exceeding typical expectations for pancreatic and colorectal cancers in this setting. If validated in larger trials, this approach could become a scalable immunotherapy option to reduce relapse and extend survival for patients with KRAS-driven cancers. The strategy also highlights the broader potential of mutation-targeted cancer vaccines to generate precise, durable anti-tumor immunity without the time and cost of fully personalized manufacturing.
Source: nature

Leave a Comment