6 Minutes
A surprising microbe in your gut — one that makes methane — may change how many calories your body extracts from food, especially from fiber. New research from Arizona State University shows that people whose microbiomes produce more methane can absorb more energy from the same high-fiber meals than people with low methane production.
Methane in the gut: the invisible calorie amplifier
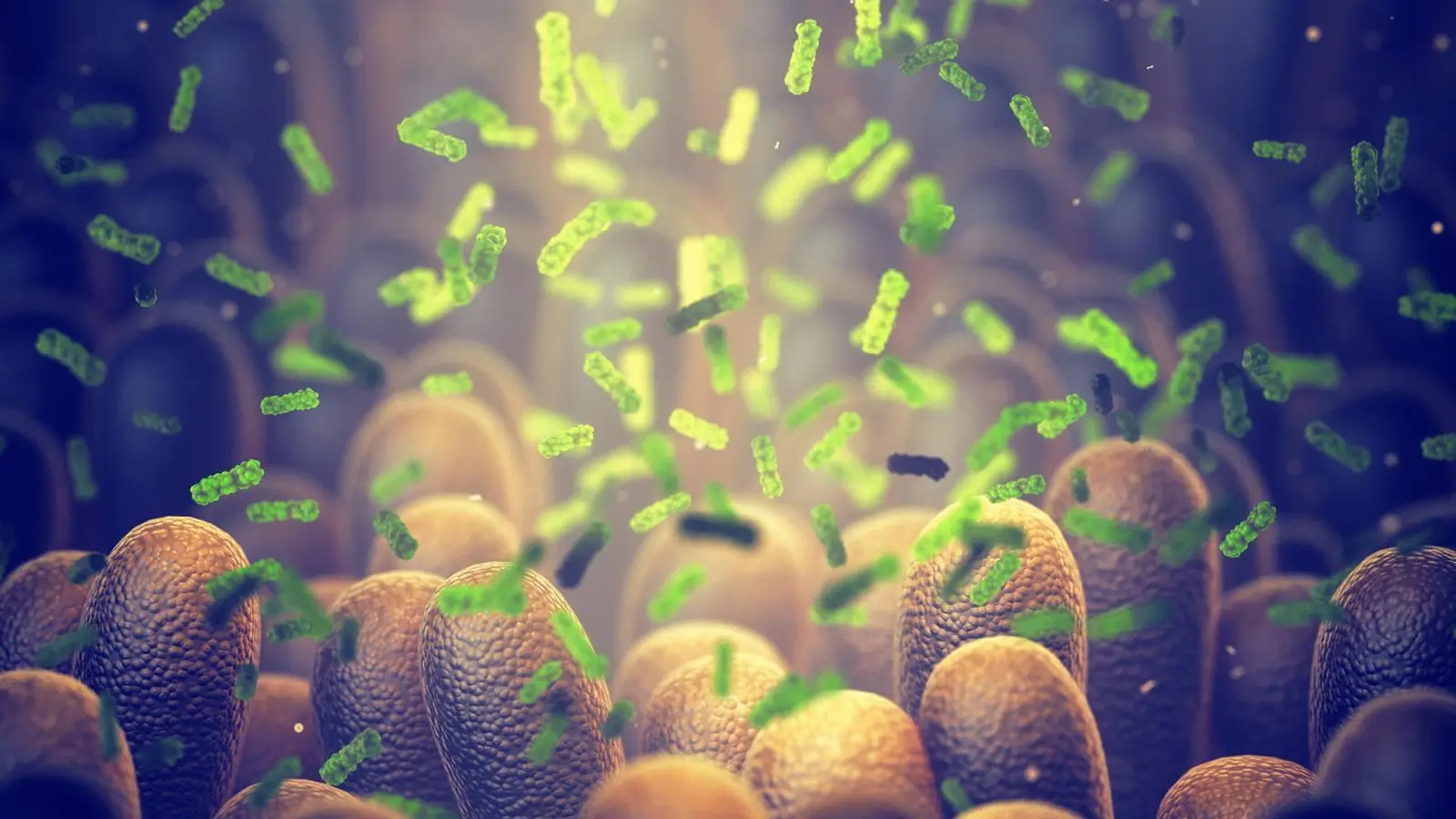
We tend to think of digestion as a human process, but much of the work happens courtesy of trillions of microbes living in our colon. These microbes ferment dietary fiber into short-chain fatty acids (SCFAs) — molecules our bodies can use for energy. During this fermentation they also produce hydrogen, which can build up and slow microbial activity unless other organisms consume it.
Enter methanogens: a group of archaea (microbe relatives of bacteria) that use hydrogen and produce methane in the process. The body itself doesn’t make methane — only these microbes do — so methane output can act as a signpost for a microbiome that’s particularly efficient at converting fiber into usable calories.
“The human body itself doesn’t make methane, only the microbes do. So we suggested it can be a biomarker that signals efficient microbial production of short-chain fatty acids,” says Rosy Krajmalnik-Brown, director of ASU’s Biodesign Center for Health Through Microbiomes and corresponding author on the study.

How the study measured metabolism — beyond a single breath test
Previous research often relied on breath tests to estimate methane, but the ASU-led team used a more thorough approach. Researchers partnered with the AdventHealth Translational Research Institute to place volunteers inside a whole-room calorimeter — a sealed, hotel-like chamber that tracks metabolic rate and all gases released over several days. Participants stayed in the calorimeter for six days while researchers continually recorded methane emissions and energy expenditure.
This method captures total methane output — from breath and other emissions — and ties it directly to metabolic measurements and stool and blood analyses. Those additional samples allowed the team to measure SCFA levels and map which microbes were active under different diets.
Two diets, different responses
Every participant ate two controlled diets in the experiment: a low-fiber, processed-food style diet and a high-fiber, whole-foods diet. Both menus were matched for macronutrient proportions (carbohydrates, proteins, fats), so differences in energy absorption were linked to how the gut microbiome handled fiber rather than to calorie composition alone.
On the high-fiber diet almost everyone absorbed fewer calories than they did on the processed-food diet. But the twist: individuals whose microbiomes produced higher levels of methane absorbed more calories from the high-fiber diet than participants with low methane output. In short, methane-producing microbiomes appeared more efficient at turning fiber into absorbable energy.
“That difference has important implications for diet interventions. It shows people on the same diet can respond differently. Part of that is due to the composition of their gut microbiome,” explains Blake Dirks, lead author and graduate researcher at ASU’s Biodesign Center. He is also a PhD student in the School of Life Sciences.
Why this matters for nutrition and weight
These findings don’t mean fiber is bad. Across the board, high-fiber diets still led to fewer calories absorbed compared with the processed-food diet. The key takeaway is that the exact amount of calories salvaged from fiber varies with a person’s microbial community. That variability could help explain why two people eating identical high-fiber meals end up with different caloric intakes once fermentation in the colon is taken into account.
Understanding methane’s role could inform personalized nutrition: clinicians might one day tailor diets based on whether a patient’s gut hosts methanogens that promote extra energy harvest. The research team also notes applications for weight-loss strategies and metabolic health, especially if future studies examine people with obesity or diabetes.
Inside the lab: collaboration and data
The study combined microbial ecology, clinical translational science, and precise energy-balance measurement. Karen D. Corbin, an associate investigator at the AdventHealth institute and co-author, highlighted the value of interdisciplinary work: “The combination of precise measures of energy balance through whole-room calorimetry with ASU’s microbial ecology expertise made key innovations possible.”
Blood and stool analysis showed that higher methane production correlated with higher SCFA production and absorption — biochemical evidence that methanogens help keep microbial fermentation going by removing excess hydrogen. That chain of interactions appears to increase the energy yield from dietary fiber.
Implications and open questions
This study is an early step toward integrating microbiome profiles into dietary advice. Important questions remain: How stable are folks’ methane-producing communities over time? Can diet, probiotics, or other interventions change methanogen levels meaningfully? And how do these dynamics differ in people with metabolic disease?
Participants in the ASU study were generally healthy, and the experiment didn’t aim for weight loss, though a few people lost some weight on the high-fiber plan. The research team is eager to test targeted diets intended for weight change and to study populations with metabolic conditions.
Expert Insight
“This work highlights a subtle but important mechanism by which microbes influence human metabolism,” says Dr. Elena Moreno, a fictional but realistic gastroenterologist and microbiome researcher. “We sometimes oversimplify fiber as uniformly low-calorie; the picture is more nuanced. Microbial partners determine how much of that fiber ends up as usable energy. Recognizing that variability could make nutritional recommendations more effective.”
The study frames methane not as a curiosity, but as a measurable biomarker that connects microbial ecology to human energy balance. That link opens new pathways for personalized diet design and metabolic research — a promising area for clinicians, nutritionists, and microbiome scientists alike.
Source: scitechdaily
Comments
skyspin
Is this even true? Whole-room calorimeter sounds legit, but can diet or probiotics really change methanogen levels longterm? idk, curious.
bioNix
Wait, so my gut farts might actually be boosting calories? wild. If methanogens up SCFAs that could explain why diets feel so different person to person. Hmm

Leave a Comment