4 Minutes
Scientists have pinpointed a single gene variant that causes an exceptionally rare form of diabetes in newborns — a discovery that sheds new light on how insulin-producing beta cells develop and fail. The mutation disables beta cells and can lead to their death, explaining a troubling trio of symptoms seen in affected infants.
One gene, a devastating childhood syndrome
An international research team traced neonatal diabetes in six infants to mutations in the TMEM167A gene. These babies also had microcephaly (an abnormally small head size) and, in five of the six cases, epilepsy — a constellation of features already recognized as MEDS (microcephaly, epilepsy, and diabetes syndrome). Until now, only two genes (IER3IP1 and YIPF5) were definitively linked to MEDS. The new findings establish TMEM167A as a third genetic cause.
How TMEM167A undermines insulin production
TMEM167A is active in both the pancreas and the brain across humans and mice — a distribution that helps explain why mutations affect both organs. To study the mutation’s effects, researchers edited human pluripotent stem cells, replacing the normal TMEM167A gene with the variant found in a MEDS patient. Those stem cells were then guided to become pancreatic beta cells, the specialized cells that produce and secrete insulin.
Development looks normal — function does not
Surprisingly, beta cells that carried the TMEM167A variant formed in appearance and number, but they were functionally defective. When exposed to glucose, these cells failed to release insulin as healthy beta cells do. The malfunction traced to stress in the endoplasmic reticulum (ER) — the cell’s protein-folding and transport network. Persistent ER stress triggered pathways that ultimately killed the beta cells.
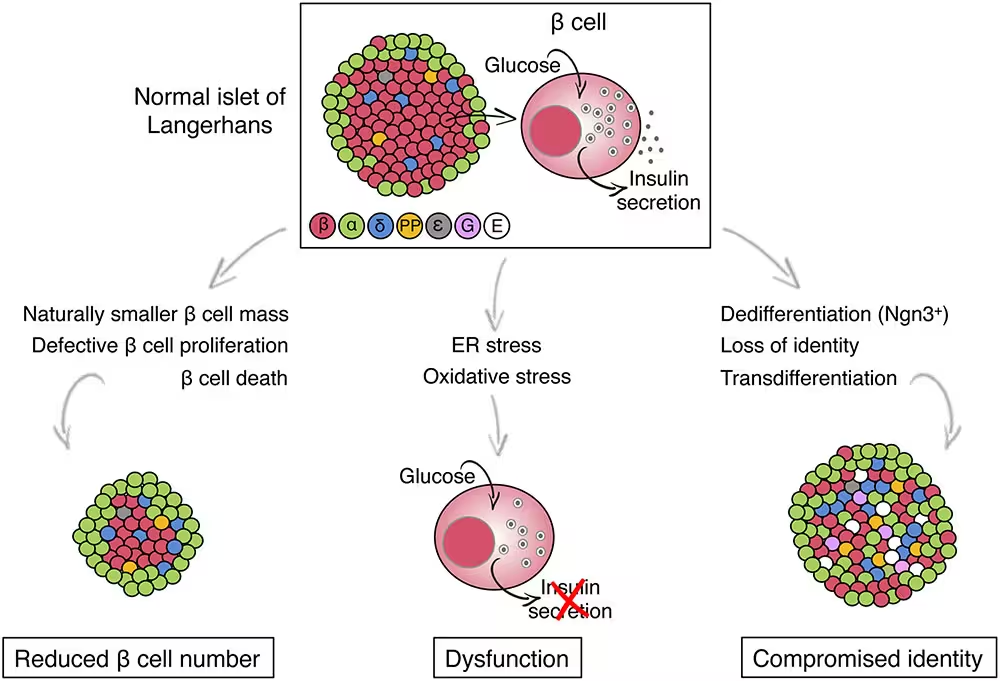
“The ability to generate insulin-producing cells from stem cells has enabled us to study what is dysfunctional in the beta cells of patients with rare forms as well as other types of diabetes,” explained diabetologist Miriam Cnop of the Free University of Brussels. “This is an extraordinary model for studying disease mechanisms and testing treatments.”
Why this matters beyond rare disease
Although MEDS is extremely rare (only about 11 cases recorded before this study), the cellular problems — ER stress, failed insulin secretion, and beta cell loss — mirror processes seen in common forms of diabetes, such as type 2. That overlap means insights from TMEM167A could inform broader diabetes research, from identifying therapeutic targets to improving stem-cell-based beta cell replacement strategies.
“Finding the DNA changes that cause diabetes in babies gives us a unique way to find the genes that play key roles in making and secreting insulin,” said molecular geneticist Elisa de Franco at the University of Exeter. “Our work clarifies how a little-known gene, TMEM167A, is crucial for insulin secretion.”
Looking ahead: diagnosis and potential therapies
Clinically, recognizing TMEM167A mutations allows for definitive genetic diagnosis of MEDS cases, which can guide family counseling and neonatal care. On the research front, the stem-cell model provides a testbed for compounds that might reduce ER stress or bolster insulin secretion. While translational therapies remain years away, the study opens a direct path from gene discovery to mechanistic understanding — and eventually, targeted interventions.
Source: sciencealert

Leave a Comment