5 Minutes
Overview
Ozempic and similar medications have transformed treatment for type 2 diabetes and obesity by activating the GLP‑1 (glucagon‑like peptide‑1) pathway to reduce appetite and improve blood sugar control. New laboratory research from Marshall University suggests that metabolites produced by gut microbes — derived from the dietary amino acid tryptophan — can stimulate the gut to produce more GLP‑1 by increasing the number of enteroendocrine cells (EECs). These findings point to a potential microbial or dietary strategy that complements pharmaceutical GLP‑1 receptor agonists such as semaglutide (marketed as Ozempic).
Scientific background: EECs, GLP‑1 and the microbiome
Enteroendocrine cells are specialized hormone‑secreting cells in the intestinal lining that synthesize GLP‑1, a peptide hormone essential for regulating glucose metabolism and satiety. Reduced EEC abundance and impaired GLP‑1 production have been observed in some forms of obesity, suggesting a mechanistic link between gut cell composition and metabolic disease. The gut microbiome metabolizes dietary compounds into bioactive molecules such as indole, which can influence epithelial cell behavior and immune signaling.
Methods: organoids and animal models
The Marshall University team combined experiments in rodent models with studies using intestinal organoids — three‑dimensional mini‑gut cultures grown from stem cells. These complementary approaches allowed researchers to track how tryptophan supplementation and microbial metabolites affect EEC differentiation and GLP‑1 production in controlled environments while observing systemic metabolic effects in animals.
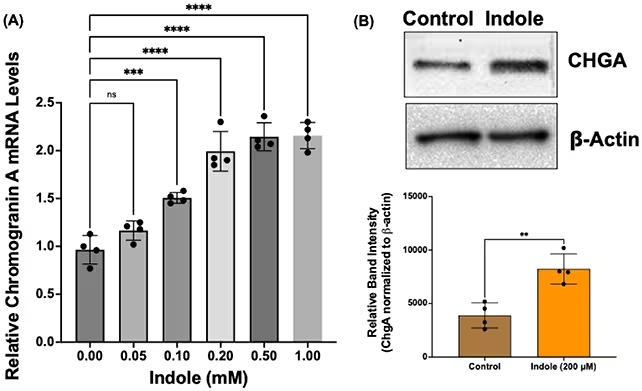
Key discoveries and mechanisms
The study found that increasing dietary tryptophan or exposing gut tissues to indole promoted the generation of new EECs, which in turn raised endogenous GLP‑1 levels. Mechanistically, the researchers identified the aryl hydrocarbon receptor (AhR) as a critical cell receptor that mediates the response to microbial tryptophan metabolites. Activation of AhR appears to trigger signaling pathways that favor EEC differentiation from intestinal progenitor cells.
Why this matters
By restoring or boosting the population of GLP‑1–producing cells, microbial metabolites could provide a more physiologic means of enhancing GLP‑1 signaling compared with exogenous drugs. "This points to a potential therapeutic strategy that leverages the gut microbes to improve metabolic outcomes in obesity," says Alip Borthakur, biochemist in the Department of Clinical & Translational Sciences at Marshall University. The authors also note in their paper that "the molecular players and signaling pathways involved in the regulation of EEC differentiation could be different in the normal and obese conditions," underscoring the need to understand context‑specific responses.
Implications, limitations and next steps
Dietary tryptophan occurs naturally in poultry, eggs, dairy, and certain seeds; the research suggests that targeted dietary supplements, engineered probiotics, or metabolite‑based therapeutics could be developed to increase local indole production in the gut and thereby elevate GLP‑1 output. However, translating these findings to human therapies will require careful clinical testing. Rodent physiology and organoid models are powerful tools but cannot capture all aspects of human metabolism, host‑microbe interactions, or long‑term safety.
Future research priorities include: (1) confirming the effect in human tissues and clinical trials, (2) mapping the precise signaling cascade downstream of AhR in human EEC differentiation, and (3) determining optimal delivery methods — diet, probiotic strains, or small‑molecule AhR modulators — that safely amplify beneficial microbial metabolism without unintended side effects.
Related technologies and research avenues
This work intersects with several active areas of biomedical research: microbiome engineering, metabolomics, organoid platforms for translational research, and drug discovery targeting nuclear and ligand‑activated receptors like AhR. Combining these tools could accelerate development of non‑pharmaceutical or adjunct strategies to enhance endogenous GLP‑1 production.
Conclusion
Marshall University’s study offers compelling preclinical evidence that gut microbial metabolites derived from dietary tryptophan can increase enteroendocrine cell numbers and GLP‑1 production through AhR‑mediated signaling. While not an immediate substitute for GLP‑1 receptor agonists such as semaglutide, this microbiome‑centered approach could eventually provide a complementary or alternative strategy to harness the body’s own hormone production for metabolic health. The next imperative is rigorous human research to validate efficacy, safety, and practical delivery methods for translating these insights into therapies.
Source: mdpi

Leave a Comment