5 Minutes
a rare genetic anomaly with outsized antiviral effects
A rare human genetic variant — a deficiency in the interferon-stimulated gene 15 (ISG15) — appears to keep antiviral defenses on low-level constant alert, producing mild persistent inflammation yet conferring broad resistance to many common viruses. Columbia University immunologist Dusan Bogunovic first described this unusual phenotype more than a decade ago. Patients with ISG15 deficiency reported routine exposures to influenza, measles, chickenpox and mumps without experiencing the typical illnesses those infections cause. Detailed laboratory analysis showed that their antiviral proteins never fully revert to a resting state, maintaining a continuous, low-grade antiviral program.
This discovery suggested a provocative idea: if the ISG15-deficient state could be reproduced transiently and safely in other individuals, it might provide broad-spectrum antiviral protection against current and emergent viral threats. That concept has now been tested preclinically using an approach analogous to mRNA vaccine technology to temporarily mimic the ISG15-deficient immune profile in lab animals.
The experiment: reproducing ISG15 deficiency with a transient mRNA-based approach
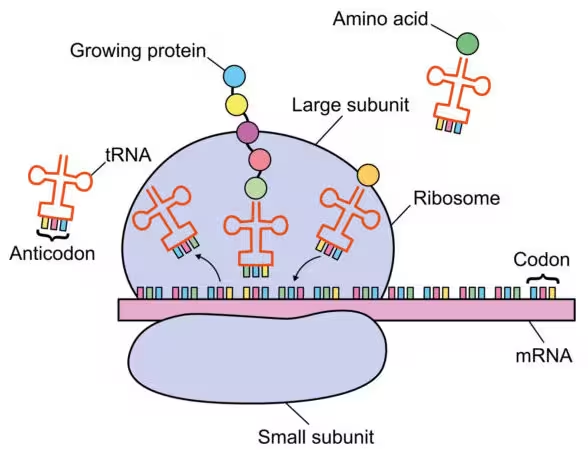
Researchers delivered instructions to target cells that reduced ISG15 activity while simultaneously increasing expression of a set of 10 proteins identified as principal mediators of the antiviral effect. These proteins act at multiple stages of the viral life cycle, disrupting entry, replication, and assembly for diverse viruses. In cell culture, the team reports they have not yet identified a virus that can overcome the therapy's defenses. In vivo, mice and hamsters treated with the transient ISG15-suppressing regimen produced higher levels of the protective proteins and showed reduced viral replication after challenge with SARS-CoV-2.
Mechanism and duration
ISG15 normally modulates interferon-driven antiviral programs. Its deficiency lifts a brake on multiple interferon-stimulated genes, creating a sustained, low-level antiviral state. The therapy intentionally induces a modest increase in the 10 key antiviral proteins for a short window — reported protection lasted up to four days in the treated animals — minimizing inflammation compared with lifelong ISG15-deficient patients while still providing meaningful viral restriction.
Outcomes and immune interactions
Treated animals showed restricted viral replication without apparent broad impairment of other immune functions. Bogunovic and colleagues emphasize the approach produces only a small, time-limited increase in these proteins, sufficient to blunt infection but not to trigger the chronic inflammatory problems observed in people with genetic ISG15 deficiency. The team proposes the technique as a potential stopgap tool: short-term, nonspecific antiviral protection for frontline workers or populations at high immediate risk during the early phase of a pandemic, before pathogen-specific vaccines are available.
Implications, challenges and future prospects
If validated and adapted for humans, transient ISG15 suppression could become part of pandemic preparedness portfolios, providing broad-spectrum antiviral coverage against novel respiratory viruses or other emerging threats. The approach aligns with broader interest in host-directed antivirals — strategies that make the host environment less permissive to pathogens rather than targeting each virus directly.
Yet significant hurdles remain. The primary technical barrier is targeted and safe delivery: getting nucleic acids (mRNA or similar constructs) to the specific tissues or cell types that need protection without off-target effects. As Bogunovic notes, "Once the therapy reaches our cells, it works, but the delivery of any nucleic acid, DNA or RNA, into the part of the body you want to protect is currently the biggest challenge in the field." Social and political resistance to mRNA technologies in some regions could also slow adoption and deployment.
Expert Insight: Dr. Priya Sharma, infectious disease epidemiologist: "A transient, host-directed antiviral could be a game changer for surge capacity and early outbreak response. The short duration is a limitation for long-term prevention, but for protecting healthcare workers or vulnerable populations during peak exposure it could buy crucial time while specific vaccines are developed. Addressing delivery and safety will determine whether this concept can move from promising preclinical results to a practical tool."
Conclusion
Recreating the antiviral state observed in rare ISG15-deficient individuals using transient, mRNA-like technology produced short-term, broad antiviral protection in mice and hamsters. The approach targets host antiviral proteins rather than the virus itself and may be useful as an immediate, nonspecific defense during early pandemic stages. However, delivery challenges, safety considerations, and social acceptance of nucleic-acid therapeutics must be resolved before human use. Continued research will clarify efficacy against diverse pathogens and the feasibility of translating this strategy into a deployable public-health intervention.
Source: science

Leave a Comment