5 Minutes
Breakthrough: storing multiple charges with light
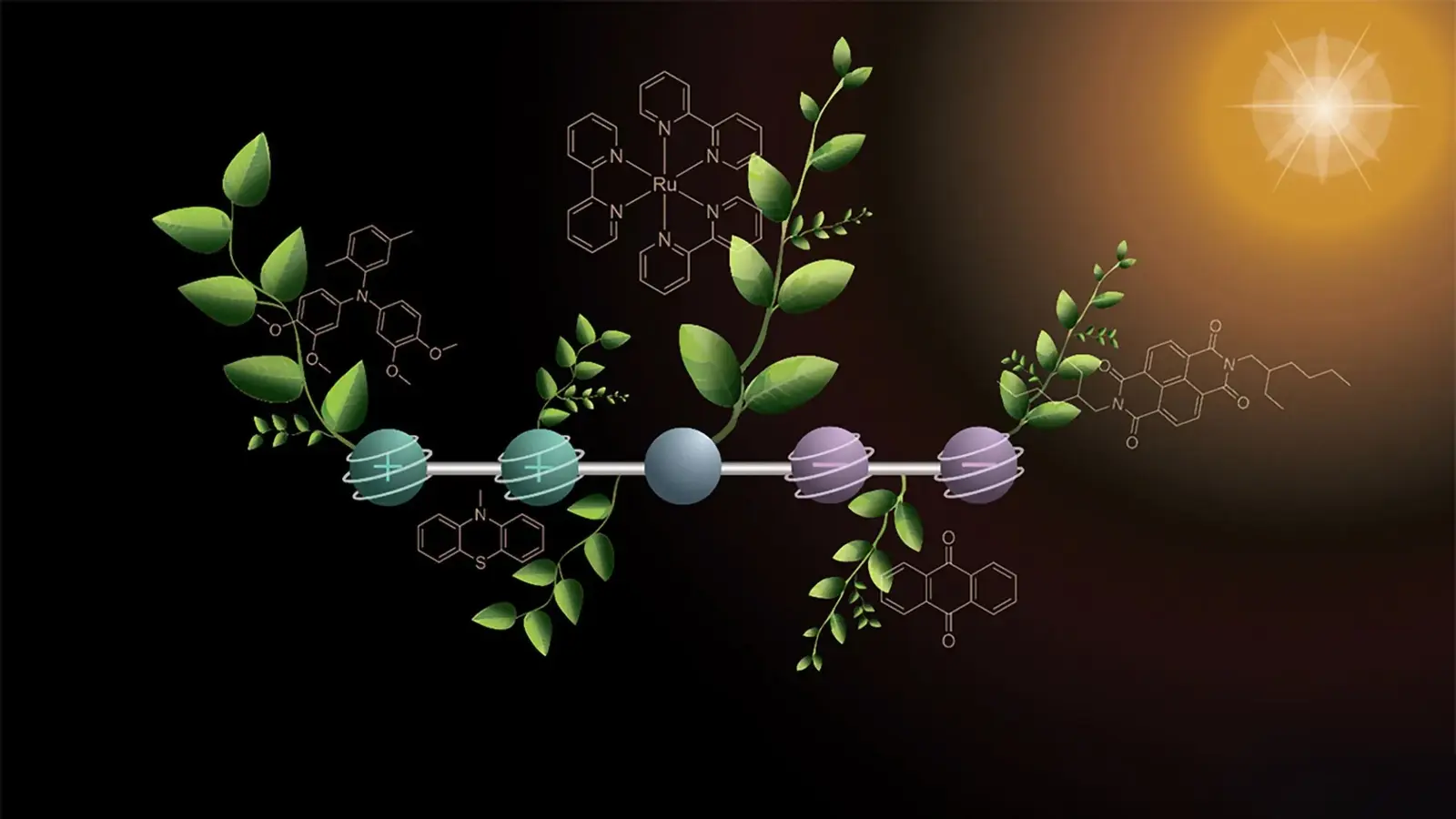
As with natural photosynthesis, the new molecule temporarily stores two positive and two negative charges. Credit: Deyanira Geisnæs Schaad
A research team at the University of Basel in Switzerland has designed a synthetic molecule that, when illuminated, can accumulate two positive and two negative charges simultaneously. Published in Nature Chemistry, the work by Professor Oliver Wenger and doctoral researcher Mathis Brändlin represents an intermediate but critical advance toward converting sunlight into carbon-neutral fuels such as hydrogen, methanol or synthetic hydrocarbons.
Photosynthesis in plants captures sunlight and channels that energy into chemical bonds, turning carbon dioxide and water into energy-dense sugars. Artificial photosynthesis aims to copy that energy conversion to produce "solar fuels" — fuels whose combustion re‑releases only the CO2 originally consumed during synthesis, making them carbon-neutral. One of the central technical challenges is temporary storage of multiple charges produced by photon-driven reactions so they can be used to power multi-electron chemical steps such as water splitting.
How the molecule captures and stores charges
Modular five-part architecture
The Basel molecule is a linear assembly of five functional units, each tuned for a specific role. A central light-absorbing component initiates electron transfer when it absorbs a photon. Two electron-donor segments on one end become oxidized (positively charged) when they release electrons, while two electron-acceptor segments on the opposite end become reduced (negatively charged) when they take up electrons. The spatial separation of oxidized and reduced sites prevents immediate recombination and enables temporary storage of the four charges.
This intermediate charge storage is essential for driving chemical reactions that require multiple electrons, for example splitting water into hydrogen and oxygen or reducing CO2 into energy-rich molecules.

Stepwise light activation and operation in low-intensity light
To build up four charges without destroying the molecule or causing uncontrolled reactions, the researchers used a stepwise two-photon approach. A first pulse of light triggers one round of electron transfer, producing one positive and one negative charge that migrate to opposite ends of the molecule. A second, time-delayed flash repeats the process, yielding a total of two positive and two negative charges held within the same molecular framework.
"This stepwise excitation makes it possible to use significantly dimmer light. As a result, we are already moving close to the intensity of sunlight," said Mathis Brändlin, noting that previous strategies often required high-intensity laser pulses that are far removed from realistic solar conditions. Importantly, the stored charges remain stable long enough to be available for subsequent chemical transformations.
Implications for solar fuels and future research
The Basel study addresses a mechanistic gap in artificial photosynthesis: how to reliably accumulate multiple charges on a single molecular scaffold under realistic illumination. Stabilized, spatially separated charges can be fed into catalytic centers to drive multi-electron reactions needed for fuel production. While the molecule itself does not yet constitute a complete artificial photosynthetic system, it provides a modular design principle for coupling light capture, charge storage, and catalysis.
Limitations remain. The current experiments demonstrate controlled charge accumulation in solution under laboratory conditions; integrating such charge-storing motifs with robust catalysts for water oxidation or CO2 reduction, and scaling to practical efficiencies, are next steps. Long-term photostability, material cost, and system integration will determine whether this approach can underpin industrial-scale solar fuels.
Expert Insight
Dr. Elena Morales, a chemical engineer specializing in solar fuels (fictional), comments: "This work is an elegant demonstration of photon management at the molecular scale. The ability to sequentially load charges with low-intensity light is crucial for moving from laser-driven demonstrations to sunlight‑compatible systems. The next major challenge will be marrying these charge reservoirs to durable catalysts that can perform the demanding chemistry of water splitting or CO2 conversion without rapid degradation."
Related technologies and outlook
Researchers worldwide are exploring complementary approaches: semiconductor photoelectrodes, molecular catalysts, and hybrid bio-inspired systems. The Basel molecule adds a promising design motif for molecular photochemistry and could be combined with heterogeneous catalysts or integrated into photoelectrochemical cells. Progress along these parallel tracks increases the chances of delivering practical solar fuel technologies that contribute to a low-carbon energy economy.
Conclusion
The University of Basel team has demonstrated a light-activated molecule that can temporarily store two positive and two negative charges using a stepwise two-photon method. This advance narrows a key gap between laboratory photochemistry and functional artificial photosynthesis by showing charge accumulation under relatively low light intensities and with sufficient stability for downstream chemistry. While further work is needed to attach catalytic reactions and scale the approach, the study provides a clear molecular strategy to help transform sunlight into carbon-neutral fuels.
Source: sciencedaily

Leave a Comment