3 Minutes
Researchers are developing a telomere-specific antioxidant strategy designed to preserve the ends of chromosomes in T cells and improve immune function. Building on work in mouse models, the team is now adapting the approach for human T cells with the long-term goal of testing safety and efficacy in clinical trials. The effort bridges cell biology, immunology, and translational medicine, with potential implications for cancer immunotherapy and post-chemotherapy recovery.
Scientific background and experimental rationale
What are telomeres and why they matter
Telomeres are repetitive DNA sequences that cap chromosome ends and protect genomic integrity. Each time a cell divides, telomeres shorten, and they are also vulnerable to oxidative damage. In immune cells such as T lymphocytes, shortened or damaged telomeres can reduce proliferative capacity and impair antiviral and anti-tumor responses.
Telomere-specific antioxidants
Unlike general antioxidants, telomere-targeted compounds are engineered to concentrate protective activity at telomeric DNA and associated shelterin proteins. In preclinical mouse experiments, investigators visualized telomeres with fluorescent markers and showed that targeted antioxidant treatment reduced telomeric damage in T cells, preserving function during repeated activation.
Clinical translation, implications, and ongoing questions
The research team is adapting the telomere-specific antioxidant approach for human T cells ex vivo, with the intention of moving toward first-in-human studies. Key translational steps include optimizing delivery to primary human T cells, confirming that telomere protection restores proliferation and cytotoxicity without promoting genomic instability, and developing manufacturing and safety protocols required for regulatory review.
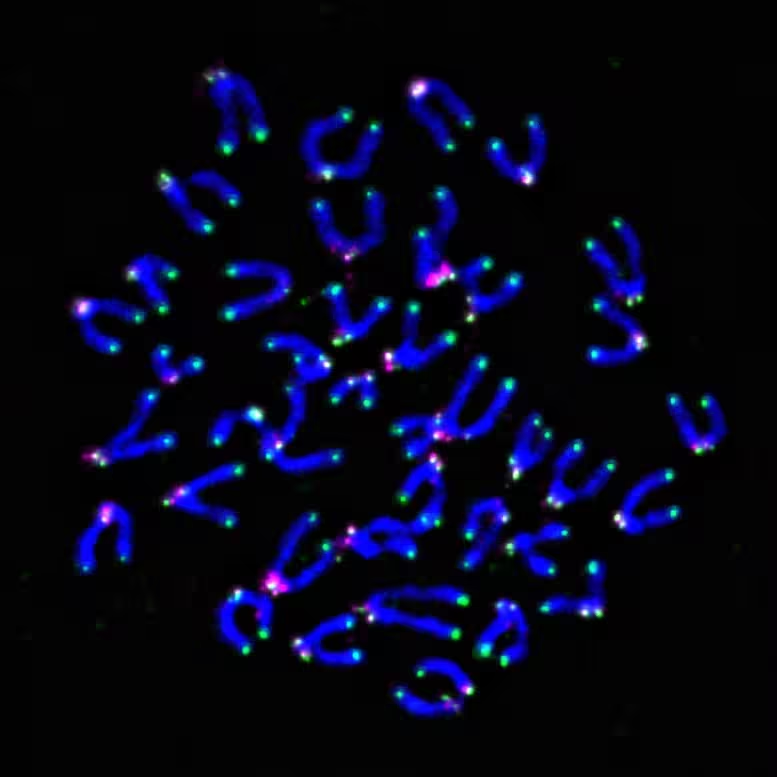
One pressing clinical question is how standard cancer treatments affect telomere integrity in immune cells. Chemotherapy and radiation generate oxidative stress that can damage telomeres in T cells, potentially blunting response to subsequent immunotherapies such as immune checkpoint inhibitors or CAR T cells. The new lab led by Dayana Rivadeneira will investigate whether protecting telomeres can improve immune recovery after chemotherapy and increase the proportion of patients who benefit from immunotherapy.
Potential challenges include ensuring long-term safety, avoiding undesirable effects on non-immune tissues, and demonstrating robust clinical benefit in heterogeneous patient populations. If successful, telomere-focused interventions could become a complementary strategy to enhance anti-cancer immunity and improve outcomes for patients receiving cytotoxic therapies.
Expert Insight
Dr. Maria Chen, an immunologist not involved in the study, notes that therapies targeting cellular aging mechanisms are an emerging frontier in cancer care. She says that telomere-focused antioxidants are promising because they act on a fundamental aspect of cell biology, but careful clinical evaluation will be essential to balance immune rejuvenation against potential risks of altering cell proliferation controls.
Conclusion
Telomere-specific antioxidants represent a targeted approach to protect T cell function by preventing telomeric damage. Early preclinical results support translation to human cells, and current work aims to determine whether this strategy can improve immune responses after chemotherapy and enhance the effectiveness of immunotherapy. Ongoing studies will clarify safety, delivery, and clinical benefit as the research advances toward trials.
Source: scitechdaily

Leave a Comment