5 Minutes
New evidence links Earth's oxygen to rust on the Moon
Recent laboratory work suggests that the surprising presence of hematite — an iron oxide commonly known as rust — at the lunar poles is driven not by local lunar chemistry but by oxygen escaping from Earth. Researchers simulated the particle environment the Moon encounters when it travels through Earth's magnetotail and found oxygen ions can oxidize certain iron-bearing materials in lunar soil, producing hematite in patterns that match orbital observations.
Scientific background: why hematite on the Moon is puzzling
Hematite (Fe2O3) forms when iron loses electrons (oxidation) in the presence of oxygen and often water. The Moon, however, lacks a substantial atmosphere and has only an ultrathin exosphere with virtually no free oxygen. It is also continuously exposed to the solar wind, a stream of hydrogen-rich plasma that chemically reduces surfaces by supplying electrons — the opposite of oxidation. These conditions make the discovery of hematite on the near side and near the poles unexpected.

An enhanced map of the hematite distribution on the near side of the Moon. (Shuai Li)
One proposed explanation invokes Earth. As the Sun drives the solar wind, Earth's magnetosphere is stretched into a long magnetotail trailing the planet. That magnetotail intermittently carries particles from Earth's upper atmosphere — including oxygen ions — outward toward the Moon. When the Moon passes through the magnetotail around full Moon, it receives a pulse of terrestrial oxygen while the bulk of the solar wind is partly blocked by Earth's shadow. This creates periodic windows of higher oxygen flux and reduced hydrogen bombardment — a potential niche for oxidation.
Laboratory simulations: reproducing Earth wind on lunar minerals
To test whether Earth-derived oxygen could oxidize lunar materials, researchers led by Xiandi Zeng at the Macau University of Science and Technology performed controlled ion-irradiation experiments. They exposed analog lunar minerals — pyroxene, olivine, ilmenite, troilite, metallic iron, and an iron meteorite — to beams of energetic oxygen ions to mimic the magnetotail "Earth wind," and to beams of hydrogen ions to simulate solar wind conditions.
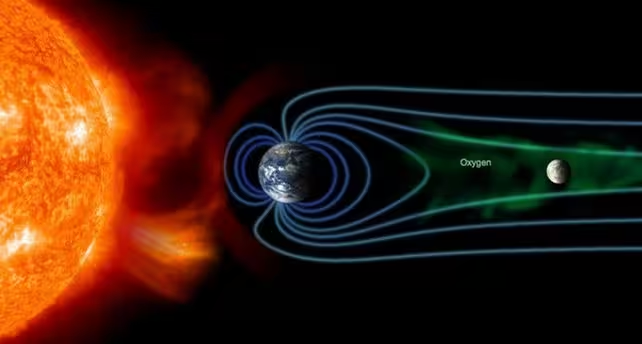
A diagram illustrating the configuration of Earth, the Moon, and the Sun that could produce hematite. (Osaka University/NASA)
The experiments showed oxygen ions can convert metallic iron, ilmenite, and troilite into hematite; metallic iron was the most susceptible. Iron-bearing silicates such as pyroxene and olivine did not produce hematite under the same conditions, indicating the oxidation process is mineral-specific. The team also found magnetite (Fe3O4) can form as an intermediate step from metal toward hematite.
Hydrogen reduction and the role of the solar wind
To evaluate whether subsequent solar wind hydrogen could undo the oxidation, the team irradiated lab-made hematite with hydrogen ions at different energies. High-energy hydrogen beams, comparable to energetic "Earth wind" particles, were able to reduce hematite back to lower-oxygen iron phases and produced water as a by-product. In contrast, lower-energy hydrogen fluxes representative of average solar wind conditions did not efficiently reverse hematite formation. These results explain why hematite can persist: episodic Earth-derived oxygen pulses can oxidize susceptible minerals while normal solar wind cannot fully reverse that oxidation.
Implications for lunar science and Earth–Moon exchange
The selective formation of hematite near lunar poles also aligns with magnetotail geometry: Earth's magnetotail preferentially directs energetic oxygen ions toward higher lunar latitudes while deflecting much of the incoming solar-wind hydrogen. Laboratory results further suggest water observed near hematite occurrences could be a reduction by-product, not necessarily a causal agent.
If terrestrial oxygen has been delivered to the Moon for billions of years, lunar hematite deposits may record changes in Earth's atmospheric oxygen over geological time, potentially back to the Great Oxidation Event roughly 2.4 billion years ago. Upcoming and recent missions that target the lunar south pole — including Chandrayaan-3's successful landing and the planned Chang'e-7 mission — will provide opportunities to sample hematite-rich regolith and test these hypotheses in situ.
Conclusion
Laboratory ion-irradiation experiments strengthen the case that escaping oxygen from Earth is the principal oxidant producing hematite on the Moon. The findings reveal a subtle but persistent chemical exchange between Earth and its satellite mediated by magnetospheric dynamics, with implications for lunar surface chemistry, the history of Earth's atmosphere, and future sample-return or in situ exploration of polar regolith.
Source: sciencealert


Leave a Comment