3 Minutes
Background: what is a hydride ion battery?
Researchers at the Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences, have demonstrated the first rechargeable hydride ion (H⁻) battery operating at room temperature. Hydride ions are hydrogen atoms that carry an extra electron; because of their extremely low mass and distinctive redox properties, H⁻ has attracted interest as an alternative charge carrier for next-generation electrochemical devices. Historically, progress has been hampered by the lack of electrolytes that combine rapid hydride mobility with thermal and electrochemical stability and good electrode compatibility.
Experiment and materials: a new core–shell hydride electrolyte
Researchers have developed the first room-temperature all-solid-state hydride ion battery. Credit: DICP
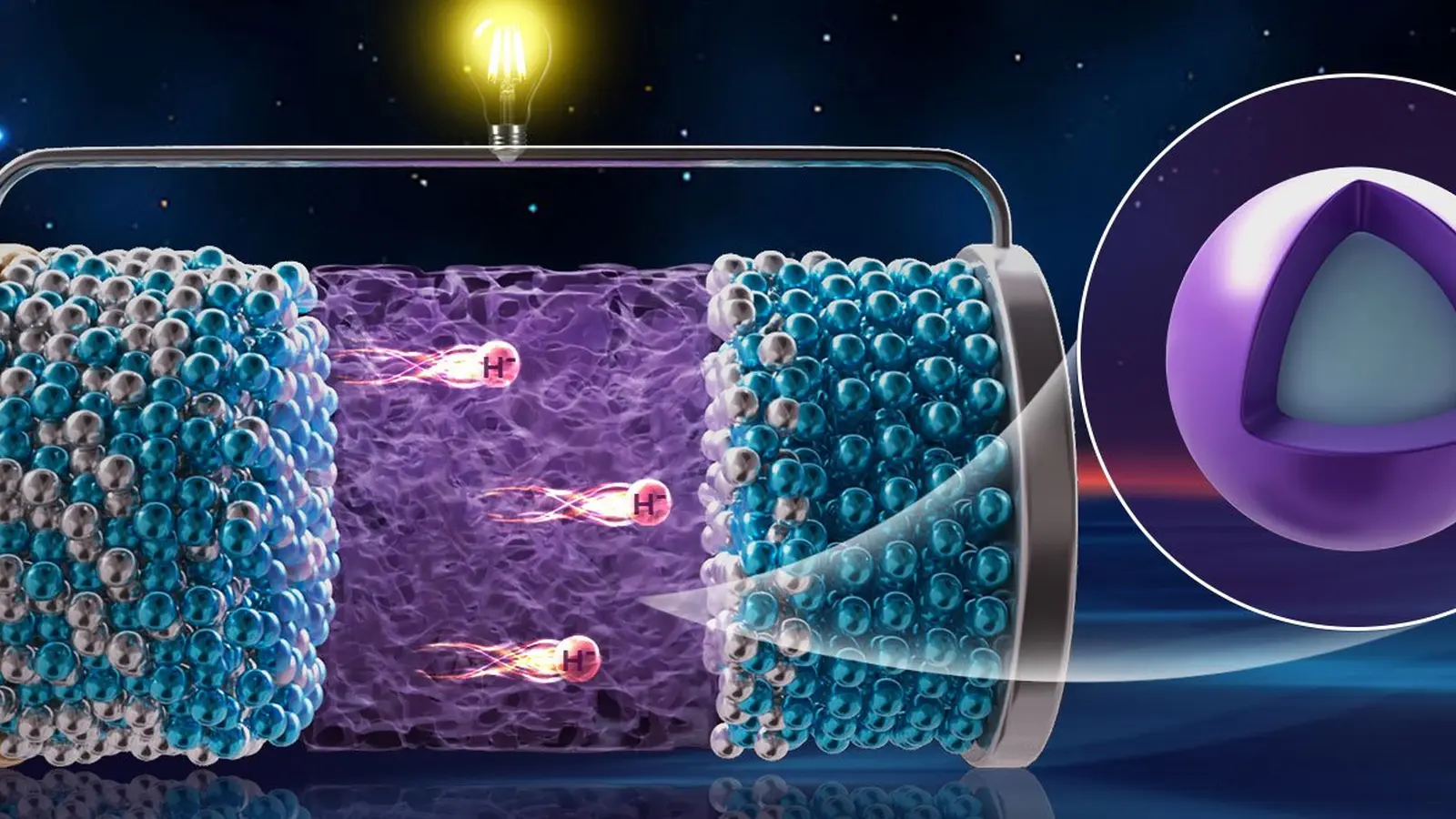
The DICP team addressed the electrolyte challenge with a heterojunction-inspired core–shell composite, designated 3CeH3@BaH2. In this design, a thin shell of barium hydride (BaH2) encapsulates a cerium hydride (CeH3) core. CeH3 provides high intrinsic hydride-ion conductivity, while the BaH2 shell contributes structural and electrochemical stability. This combination enables fast H⁻ conduction at ambient temperature while resisting degradation under typical battery operating conditions.

Battery assembly and performance metrics
Using this electrolyte, the researchers assembled an all-solid-state prototype with the cell configuration CeH2 | 3CeH3@BaH2 | NaAlH4. Sodium alanate (NaAlH4), a well-known hydrogen storage compound, served as the cathode active material. At room temperature the positive electrode exhibited an initial discharge capacity of 984 mAh g⁻1 and retained 402 mAh g⁻1 after 20 cycles. In a stacked configuration the prototype produced an operating voltage of about 1.9 V and successfully powered a yellow LED lamp, demonstrating a simple practical load test.
Key discoveries, safety, and implications
The core–shell electrolyte overcame three critical barriers: rapid hydride transport at room temperature, thermal robustness, and electrode compatibility. By using hydrogen-based charge carriers, the system also avoids metal dendrite formation — a common source of shorting and safety risk in some metal anode batteries — which could offer improved operational safety.
According to Prof. Ping Chen and colleagues at DICP, the tunable chemistry of hydride materials opens new avenues to optimize capacity, rate capability, and cyclability. While these results are an early laboratory demonstration rather than a market-ready product, they represent an important materials and design advance toward clean, efficient energy storage systems that exploit hydride ion chemistry.
Future prospects
Next steps include improving cycle life, scaling synthesis of the composite electrolyte, and integrating full-cell architectures optimized for energy density, power, and manufacturability. If those technical milestones are met, hydride-ion batteries could complement existing rechargeable chemistries for grid storage, portable devices, or niche applications where low mass and hydrogen-based electrochemistry provide unique advantages.
Source: scitechdaily

Leave a Comment