5 Minutes
Researchers report a small engineered peptide that keeps the Parkinson's-linked protein alpha-synuclein in its normal shape, preventing the misfolding and clumping thought to damage neurons. Tested in a basic worm model, the molecule resists breakdown inside cells and does not interfere with alpha-synuclein’s healthy role in neurotransmission — offering a promising, early-stage approach to stopping protein aggregation before it becomes toxic.
A tiny peptide that locks alpha-synuclein in place
Alpha-synuclein is a small neuronal protein involved in regulating dopamine and other neurotransmitters. In Parkinson's disease and related disorders, the protein can misfold and assemble into insoluble aggregates that interfere with neural communication and eventually contribute to cell death. Instead of trying to dissolve existing clumps, a team led from the University of Bath designed a peptide that binds alpha-synuclein and stabilizes its healthy conformation — essentially freezing it before it can take the wrong shape.
The approach began with a larger fragment of alpha-synuclein previously shown to reduce aggregation. The researchers trimmed that fragment down to the minimal active sequence and chemically reinforced it using lactam bridges, loops that lock the peptide’s structure and improve its resistance to cellular degradation. In worm models of Parkinson’s, this engineered peptide stopped alpha-synuclein from misfolding, reduced toxic aggregation and did so without observable cellular toxicity.
Why preventing misfolding matters for disease
There’s an important distinction between treatments that remove aggregates and those that prevent them. Aggregates may be a downstream effect of disease processes or they may actively drive progression — separating cause from consequence is a major challenge in neurodegeneration research. A preventative molecule, like the peptide in this study, aims to block the earliest event in the aggregation cascade: the initial misfolding.
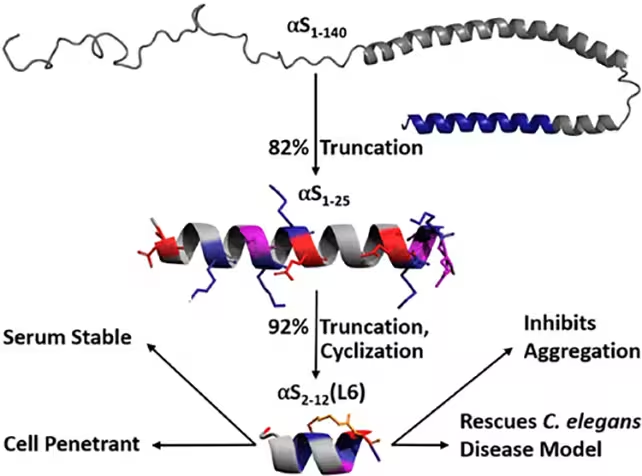
That preventive angle could be particularly useful for people identified as being at elevated risk for Parkinson's, for example through genetic screening or early biomarkers. By keeping alpha-synuclein in its functional state, cells retain normal neurotransmitter regulation while being spared the progressive buildup of toxic clumps. As the University of Bath team notes, the peptide functions inside living systems and does not disrupt alpha-synuclein’s physiological role — a key requirement for any therapy that targets an abundant, normally useful protein.
Delivery hurdles, broader implications and future directions
Despite encouraging results in worms, turning a cell-stable peptide into a human therapy raises several hurdles. Peptides can be rapidly cleared from the bloodstream, struggle to cross the blood–brain barrier, and face immune detection. The work at Bath addressed intracellular stability chemically, but delivering the molecule safely and effectively to human brain tissue remains a major engineering and pharmacology challenge.
The research team and funders emphasize that this is an early but important step. Julia Dudley of Alzheimer's Research UK, which co-funded the study, highlighted the wider relevance: similar aggregation processes underlie Lewy body dementia and Alzheimer's disease, and stabilizing native protein conformations could become a broader strategy across neurodegenerative disorders. The study appears in JACS Au (2025) and builds on prior structural mapping of alpha-synuclein interaction sites.
Expert Insight
"Designing a peptide that is both stable inside cells and selective for a disease-prone form of a protein is a difficult balancing act," says Dr. Mark Ellison, a fictional neurochemist and science communicator. "This study shows a rational-design route that reduces aggregation without knocking out the protein’s normal function — that’s exactly the kind of specificity we need as we move from model organisms toward clinical testing."
Next steps for the research include testing in mammalian models, optimizing delivery strategies (for example, nanoparticle carriers or modified peptides that cross the blood–brain barrier), and assessing long-term safety and efficacy. If those stages succeed, the peptide or related molecules could become part of preventive treatment strategies for people at high risk of Parkinson’s or other protein-aggregation disorders.
For now, the study represents a proof of principle: it’s possible to rationally design small peptides that patrol cells and block harmful protein aggregation while leaving normal biology intact — a promising blueprint for new disease-modifying approaches in neurodegeneration.
Source: sciencealert

Leave a Comment