5 Minutes
Overview and scientific context
Researchers have developed an advanced genetic test—a polygenic score (PGS)—that significantly improves early prediction of which children are at elevated genetic risk for a higher body mass index (BMI) later in life. Polygenic scores aggregate the small effects of many genetic variants across the genome to estimate predisposition to complex traits such as BMI. This type of genetic prediction is increasingly used in genetic epidemiology and precision medicine to identify individuals who might benefit from earlier or tailored interventions.
Study design, data sources and methods
The new PGS was constructed by an international consortium using genome-wide data from more than 5.1 million individuals, one of the largest training sets for a polygenic prediction to date. The team validated the score across multiple independent health cohorts that included both serial BMI measurements and genomic data for hundreds of thousands of people. By combining the PGS with conventional predictors, the researchers quantified how much of the variation in later BMI could be explained by inherited genetic variation at different ages and across ancestries.
Key findings and implications
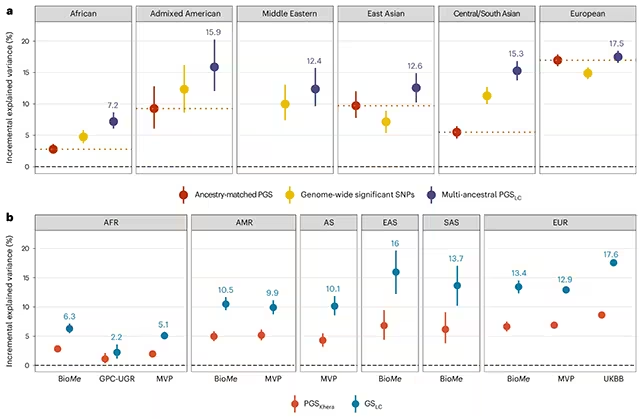
Results show the PGS can forecast a substantial share of future BMI variation at early ages. For example, the score measured at age five explained roughly 35% of the variation in BMI at age 18 in the studied European cohorts, and about 17.6% of BMI variation in middle-aged Europeans. Predictive performance, however, varied markedly by ancestry and population: in some underrepresented groups, such as rural Ugandan cohorts, the score explained far less (≈2.2%). The researchers attribute this disparity mainly to underrepresentation of African genomes in the training data and to higher genetic diversity in African populations.
Another notable observation was that individuals with a stronger genetic predisposition to higher BMI often lost more weight during the first year of structured weight-loss programs, yet they were also more likely to experience weight regain later. This pattern suggests that genetic predisposition is not strictly deterministic; people with higher polygenic risk can respond to lifestyle interventions, but long-term maintenance may be more challenging.
Limitations and ethical considerations
Limitations
While the PGS represents a marked improvement over prior scores—reportedly up to twice as accurate in some comparisons—genetics remains only one part of the risk equation. Environmental, behavioral, socioeconomic and developmental factors all shape BMI across the life course. In addition, BMI itself is an imperfect proxy for body composition and health; growing research encourages complementary measures (e.g., waist-to-height ratio, DXA-assessed fat mass) for clinical assessment.
Equity and representation
The drop in predictive power for underrepresented ancestries highlights the need for more diverse genomic datasets before polygenic tests can be equitably deployed. Without broader representation, clinical implementation risks exacerbating health disparities.
Applications, technologies and future prospects
Potential near-term applications include earlier, targeted prevention strategies: if children at higher genetic risk are identified before age five, parents and clinicians may have a longer window to promote healthy nutrition, physical activity and supportive environments. Integration with digital health tools, electronic health records and behavioural programs could enable personalised prevention pathways. Future improvements will rely on adding diverse genomic data, refining models to predict clinically meaningful outcomes (not only BMI), and testing how best to combine genetic information with social and environmental risk factors.
Expert perspective
Researchers emphasize that the new polygenic score represents a major advance toward clinically useful genetic prediction of obesity risk. They caution, however, that prediction is age- and ancestry-dependent, and that ethical deployment requires careful attention to equity, consent and potential psychosocial effects for families.
Conclusion
The new PGS offers a stronger tool for early identification of children at genetic risk for higher BMI, enabling a potentially larger window for preventive action. Yet limitations—especially reduced accuracy in underrepresented populations and the broader shortcomings of BMI as a health metric—mean it should be used cautiously and as part of a multifactorial approach that combines genetics, environment and behaviour. Continued expansion of diverse genomic data and real-world trials will determine whether these genetic predictions can be responsibly translated into routine clinical practice and public health strategies.
Source: nature

Leave a Comment