4 Minutes
Discovery: Cells Rapidly Expel Waste to Reprogram After Injury
Researchers have identified a fast, dramatic waste-removal behavior in injured cells that appears to accelerate their return to a stem cell-like state. The process—named "cathartocytosis" by the study authors—was observed in gastric tissue and is linked to paligenosis, a recently described reprogramming program through which mature cells revert to progenitor-like states to regenerate damaged tissue.
The team, led by investigators at Washington University in St. Louis and Baylor College of Medicine, describes cathartocytosis as a near-instantaneous ejection of cellular debris and organelles across the plasma membrane. Unlike the slower, intracellular digestion performed by lysosomes, this rapid purge appears to clear mature, task-specific machinery so the cell can downsize and begin proliferating to repair injury.
Mechanism and Experimental Evidence
Researchers initially expected paligenosis-associated cleanup to proceed mainly through lysosomal degradation, an internal and gradual recycling system. Instead, consistent observations of extracellular debris prompted deeper investigation. In mouse models of stomach injury, cells undergoing paligenosis frequently displayed membrane cavities and expelled intracellular material into the surrounding space—behavior the authors characterize as analogous to vomiting.
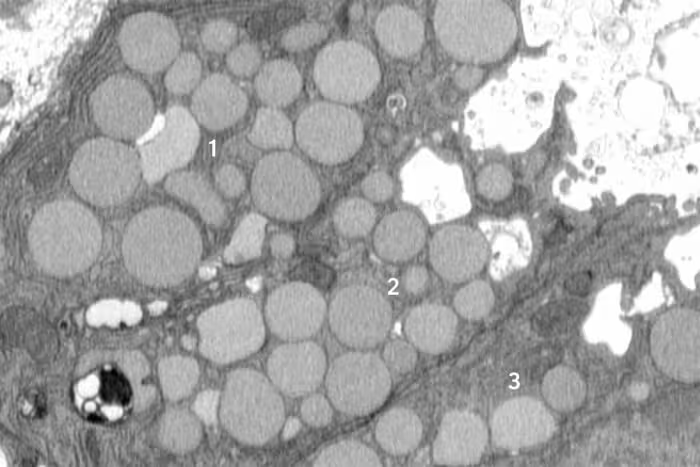
Three mouse stomach cells (numbered 1-3) are shown jettisoning cellular debris through cavities (white) that form in their membranes. (Jeffrey Brown/Washington University School of Medicine)
Using time-resolved microscopy, tissue analysis, and molecular markers for organelle clearance and membrane remodeling, the group established cathartocytosis as a reproducible component of paligenosis rather than an anomalous artifact. First author Jeffrey W. Brown, a gastroenterologist at Washington University, explains that this rapid purge helps cells overcome interference from specialized cellular machinery so they can adopt a smaller, proliferative phenotype capable of contributing to tissue repair.
Benefits, Trade-offs, and Clinical Implications
Although cathartocytosis may accelerate regeneration, senior author Jason C. Mills (Baylor College of Medicine) and colleagues warn it carries biological costs. Rapid expulsion of intracellular components can provoke local inflammation and alter the tissue microenvironment, potentially promoting mutation expansion when long-lived, mutation-bearing cells revert to a stem-like state and multiply.
The researchers point out a plausible link between repeated or chronic cathartocytosis in aging gastric cells and increased cancer risk: inflammation plus proliferation creates a permissive context for mutated clones to expand. At the same time, cathartocytosis could provide a new biomarker for precancerous changes; detecting extracellular cellular debris or membrane remodeling events might flag tissues undergoing risky reprogramming before malignant transformation.
Therapeutic strategies could aim to harness the regenerative advantage of paligenosis and cathartocytosis while mitigating oncogenic risk—for example, by promoting controlled cleanup pathways or suppressing chronic, inflammation-driven reprogramming in at-risk patients. The study is published in Cell Reports.
Expert Insight
Dr. Elena Rivera, a fictional cell biologist and science communicator with experience in regenerative medicine, comments: "This work highlights a trade-off common in biology: speed versus fidelity. Rapid reprogramming may be essential for acute repair, but if tissues continually cycle through this state—especially in older individuals—the chance for maladaptive outcomes increases. Future work should map how cathartocytosis is regulated across organs and whether we can pharmacologically steer cells toward safer recycling routes."
Conclusion
The identification of cathartocytosis expands our understanding of how mature cells retool themselves to heal injury. By quickly expelling bulky, specialized machinery, cells can adopt stem-like behaviors that speed regeneration—but this shortcut may also heighten inflammation and cancer risk when repeated or chronic. Ongoing research will be needed to determine how widespread cathartocytosis is across tissues and whether its benefits can be separated from its hazards for safer therapeutic use.
Source: sciencealert

Leave a Comment