5 Minutes
New electron-beam method converts adamantane into pristine nanodiamonds
Researchers have developed a low-pressure, electron-beam approach that transforms the carbon cage molecule adamantane (C10H16) into defect-free nanodiamonds. This technique, led by Professor Eiichi Nakamura and colleagues at the University of Tokyo, achieves diamond formation while preventing the extensive beam damage normally observed in organic materials. The discovery could expand the capabilities of electron microscopy, electron lithography, and materials synthesis.
Scientific background and why adamantane matters
Diamond synthesis has traditionally required extreme conditions: pressures of tens of gigapascals and temperatures of thousands of kelvin, or specialized chemical vapor deposition (CVD) environments where diamond may be metastable. Adamantane presents a different starting point. Structurally, adamantane shares the same tetrahedral carbon framework as diamond, making it a natural molecular precursor for building a three-dimensional diamond lattice at the nanoscale.
Converting adamantane into diamond requires targeted cleavage of C–H bonds so that C–C bonds can form between neighboring molecules and produce the rigid sp3-bonded network characteristic of diamond. Prior experimental evidence from mass spectrometry suggested single-electron ionization can induce such bond cleavage, but mass spectrometry operates in the gas phase and cannot show solid-state assembly or isolate resulting structures.
Experiment details: TEM-driven synthesis under low pressure
To observe and control the conversion, the research team used transmission electron microscopy (TEM) to irradiate submicron adamantane crystals with electrons at energies between 80 and 200 keV and sample temperatures from 100 to 296 K in vacuum. Time-resolved TEM allowed direct visualization of molecular rearrangement and polymerization as irradiation progressed over tens of seconds.
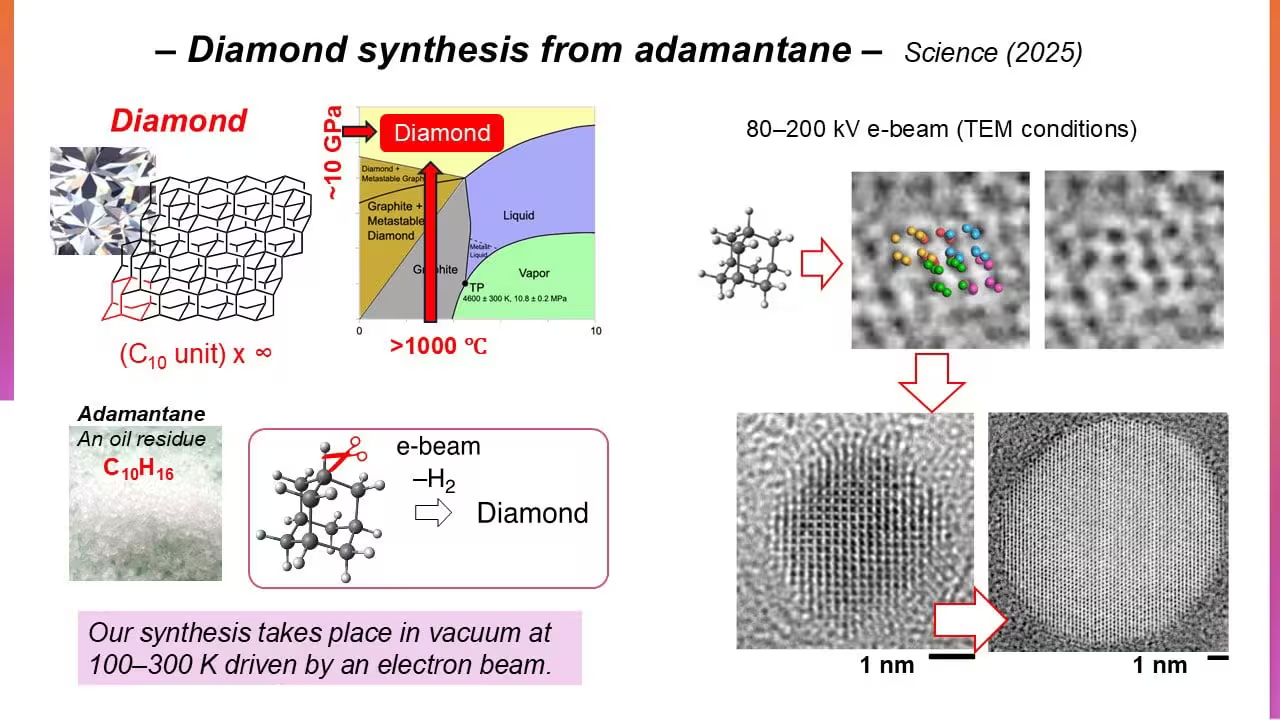
Transmission electron microscopy images show the arrangement of adamantane molecules into diamond structures under electron irradiation. Credit: Nakamura et al. CC-BY-ND
Key to the approach is sample preparation: by installing specific molecular properties and controlling irradiation parameters, the electron beam triggers targeted C–H cleavage while enabling the molecules to re-bond into a cubic diamond lattice. Under prolonged irradiation the team observed formation of spherical, defect-free cubic nanodiamonds up to about 10 nm in diameter, accompanied by the release of hydrogen gas. Other hydrocarbon precursors failed to produce the same outcome, highlighting adamantane’s unique suitability as a nanodiamond precursor.
Professor Nakamura, who combined decades of synthetic chemistry with quantum chemical modelling, explained the motivation: “Computational data gives you ‘virtual’ reaction paths, but I wanted to see it with my eyes. The common wisdom among TEM specialists was that organic molecules decompose quickly under an electron beam. My research since 2004 has been a constant battle to show otherwise.”
Key discoveries and wider implications
The team’s observations overturn a long-standing assumption: electrons do not simply destroy organic molecules; under controlled conditions they can drive well-defined chemical reactions that construct complex, ordered solids. Practical implications include:
- New routes to synthesize nanodiamonds and doped diamond quantum dots useful for quantum sensing and computing.
- Enhanced methods to study and manipulate organic reactions directly inside electron microscopes, improving in situ characterization for materials science and surface engineering.
- A possible explanation for exotic diamond formation in nature — for example in meteorites or uranium-bearing rocks — where high-energy particle irradiation could induce solid-state diamond genesis.
Future prospects and next steps
Future work will likely optimize doping strategies, scale-up pathways, and integrate this low-pressure method with surface-patterning techniques. Researchers will examine how controlled electron irradiation can produce tailored nanodiamond sizes, defect configurations, and impurity incorporation (e.g., nitrogen or silicon centers) essential for quantum applications.
If generalized to other molecular scaffolds with designed bond cleavability, the approach could open a broader class of beam-driven syntheses for hard-carbon materials and new device components.
Expert Insight
Dr. Laura Chen, a materials physicist unaffiliated with the study, comments: “This work reframes how we think about beam–matter interactions. Instead of treating electron beams solely as imaging probes, the team shows they can be precise synthetic tools. Combining molecular design with in situ TEM gives a powerful platform for making and watching materials emerge at the atomic scale.”
Conclusion
The electron-beam conversion of adamantane into defect-free nanodiamonds represents a two-decade research ambition realized: a controlled, low-pressure pathway to synthesize crystalline diamond at the nanoscale directly under an electron beam. By demonstrating that electrons can orchestrate constructive chemistry rather than indiscriminate damage, this research opens new possibilities for in situ materials synthesis, quantum dot fabrication, and the fundamental study of energetic particle-driven chemistry.
Source: scitechdaily


Leave a Comment