6 Minutes
Nobel recognition for porous crystals
Three scientists — Susumu Kitagawa (Kyoto University), Richard Robson (University of Melbourne) and Omar M. Yaghi (University of California, Berkeley) — have been awarded the 2025 Nobel Prize in Chemistry for pioneering the design and development of metal-organic frameworks (MOFs). The prize recognises their discovery of a new molecular architecture: crystalline materials built around permanent microscopic cavities that can host gases and small molecules.
The award highlights how modern materials chemistry has leveraged metal–organic coordination to create highly tuneable, porous solids. The Nobel Committee cited the transformative potential of these materials across energy, environment and medicine, and the prize sum of 11 million Swedish kronor will be shared by the three researchers.
Scientific background and the evolution of MOF chemistry
The roots of MOF chemistry go back to early work on coordination polymers in the 1950s and 1960s — linked chains of metal ions connected by organic molecules called linkers. Those early materials did not contain accessible voids, but they established the metal–organic chemistry that later enabled framework design.
In the late 1980s Richard Robson's group showed that certain coordination polymers could assemble into framework-like crystals where organic linkers formed three-dimensional cages around clusters of liquid solvent molecules. His 1980s papers described an "unusual situation in which approximately two-thirds of the contents of what is undoubtedly a crystal are effectively liquid," revealing the potential for crystals with internal space.
In the mid-to-late 1990s, Omar Yaghi's group demonstrated that some of these frameworks remain intact after the solvent is removed — a landmark discovery that dispelled the assumption that such open frameworks would collapse when emptied. Around the same time, Susumu Kitagawa showed these empty cavities could selectively adsorb gas molecules and even undergo reversible expansion and contraction as guests enter and leave, establishing function as well as form.
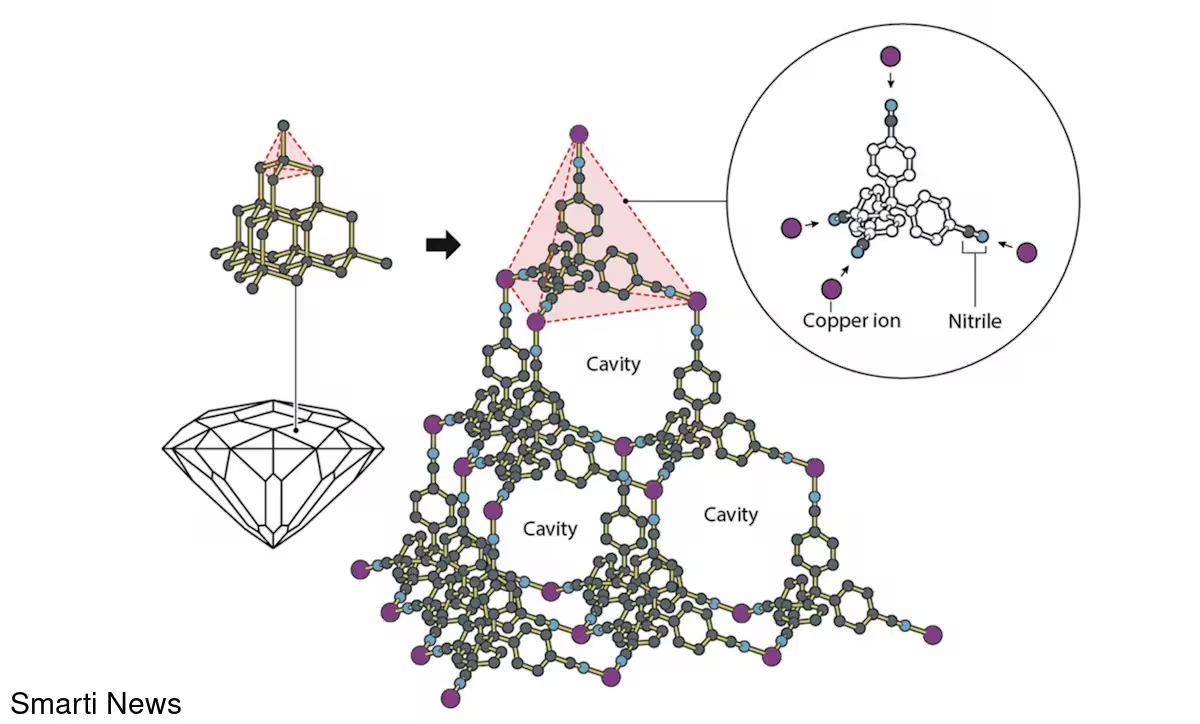
Crystal structures with in-built cavities. (Nobel Prize outreach, CC BY-SA)
These coordinated advances marked the birth of modern MOF chemistry. One notable product of this work, MOF-5 (constructed by Yaghi), combined high porosity with structural stability: a few grams of MOF-5 can possess an internal surface area comparable to that of a football pitch.
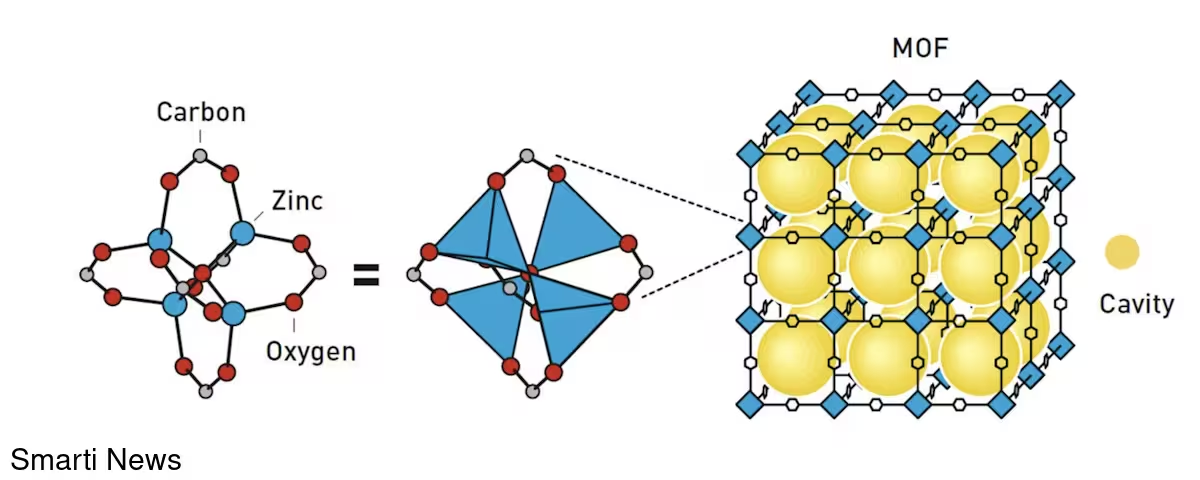
Key discoveries and practical implications
MOFs are a diverse class of crystalline, porous materials whose cavity sizes range from a few ångströms to several nanometres — ideal dimensions for hosting gases, small molecules, and even small drug payloads. Their structure is modular: chemists can swap metals or organic linkers to tune pore size, chemical affinity, and stability. This design flexibility underpins many important applications:
Gas storage and separation
MOFs can adsorb gases at much higher densities than in the free gas phase, which makes them promising for compact hydrogen storage for fuel-cell vehicles, methane storage for cleaner transport, and CO2 capture from flue gases or ambient air. Their molecular selectivity allows them to separate gas mixtures efficiently, improving carbon capture and emissions-control technologies.
Catalysis and chemical reactions
The internal cavities act as controllable microreactors: metals in the framework can serve as catalytic sites while the pores confine reactants, enhancing selectivity and efficiency. Because MOF composition is highly tunable, researchers can design frameworks specifically for particular catalytic transformations.
Water harvesting, drug delivery and energy storage
MOFs can pull water from humid air, offering strategies to harvest freshwater in arid regions. Their porous networks can encapsulate pharmaceutical molecules for controlled release and are being explored for applications in batteries, thermal energy storage, and chemical sensing. The breadth of applications continues to expand as researchers explore new compositions and composite materials.
Related technologies and future prospects
Continued research focuses on improving MOF stability, scalability and integration into devices. Key engineering challenges include producing MOFs at industrial scale, ensuring long-term durability under real-world conditions, and embedding MOFs into membranes or electrodes for practical deployment.
Several industrial pilots are already testing MOFs for CO2 capture and water capture systems. Materials-by-design approaches, combined with machine learning and automated synthesis, accelerate discovery of MOFs optimized for specific tasks such as selective separations or high-capacity hydrogen storage.
The Nobel Committee noted that these discoveries "opened up an entirely new branch of chemistry with practical consequences for climate mitigation, energy storage and resource recovery." The award both recognises decades of fundamental work and signals increased interest in translating MOF research into commercial technologies.
Expert Insight
Dr. Elena Morales, materials chemist at a national laboratory, commented: "The elegance of MOF chemistry is its modularity — by choosing different metals and organic linkers, you can design a material for a narrow, targeted function. That tunability, combined with impressive porosity, is why MOFs keep producing breakthroughs in energy and environmental applications."
Conclusion
The 2025 Nobel Prize for Chemistry honours a conceptual and technical advance: the creation of stable, porous crystalline frameworks that combine precision design with practical function. From CO2 capture and hydrogen storage to catalysis and water harvesting, metal-organic frameworks are a versatile platform at the intersection of chemistry, materials science and engineering. Decades after their discovery, MOFs remain a dynamic and rapidly expanding field with significant potential to contribute to sustainable technologies and next-generation materials.
Source: sciencealert

Leave a Comment