5 Minutes
New research indicates that Alzheimer's disease may interfere with the 24-hour genetic 'clocks' inside brain support cells, altering when key risk genes switch on and off. That disruption could change how the brain cleans up toxic proteins and maintains daily neuronal function—opening a fresh path for potential therapies.
What researchers looked at and why it matters
Scientists from Washington University School of Medicine compared gene activity across the day in brains from healthy mice and mice engineered to develop Alzheimer's-like amyloid plaques. They focused on two non-neuronal cell types: astrocytes, which provide metabolic and structural support to neurons, and microglia, the resident immune cells that clear debris. The team then cross-checked those findings against human brain tissue.
The concept at the heart of the study is the circadian rhythm—the internal timing system that coordinates sleep, metabolism and many cellular tasks over a roughly 24-hour cycle. While circadian disruption has long been associated with dementia and sleep problems in patients, this work drills down to how daily oscillations in gene expression change inside specific brain cells during disease.
Key discoveries: which genes get caught in the clock
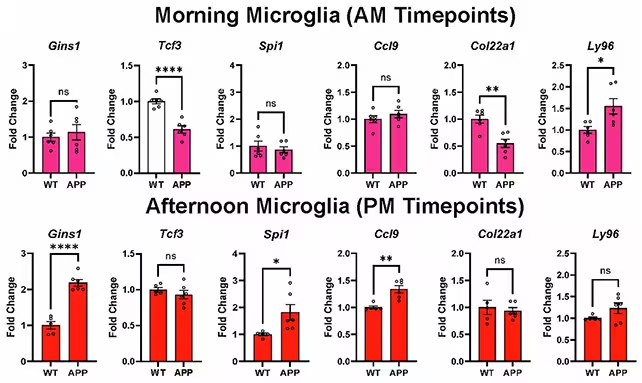
Out of 82 genes previously linked to Alzheimer's risk, investigators found that roughly half show circadian regulation in glial cells. In other words, the activity of many Alzheimer's-associated genes rises and falls on a daily schedule in healthy brains—and those rhythms are altered in the disease model.
Tracking gene expression in microglia over the course of the day revealed pronounced shifts when Alzheimer's pathology was present. These clock-driven changes could affect how glia perform core jobs such as clearing amyloid-beta and regulating inflammation—functions central to keeping the brain environment clean and functional.
Which way does causation run?
One central question remains unanswered: does amyloid buildup disrupt cellular clocks, or does a weakened clock increase the risk of plaque formation? The researchers lean toward the idea that altered circadian timing in specific cell types contributes to disease progression, but the relationship is likely bidirectional—disease biology and clock function probably amplify each other.
Why timing matters for brain cleanup and symptoms
About 20% of human genes oscillate with the circadian cycle, governing processes from digestion to tissue repair. If glial clocks go off-beat, the timing of waste clearance, immune surveillance and metabolic support may also shift—leading to impaired removal of toxic proteins and increased vulnerability to neurodegeneration.
Clinically, circadian disruption manifests in recognizable ways. Patients with Alzheimer's often experience sleep fragmentation and a worsening of confusion late in the day, a phenomenon called sundowning. The new molecular evidence ties those symptoms to tangible shifts in gene regulation inside the brain's cleaning and immune cells.
Therapeutic possibilities: resetting the brain's clocks
Because many Alzheimer's-linked genes are under circadian control, the authors suggest a novel therapeutic angle: modulate the cellular clock. That could mean strengthening rhythms in beneficial cell types, damping harmful oscillations, or selectively altering timing in microglia and astrocytes to restore efficient clearance of amyloid and reduce inflammation.
Practical approaches might include pharmacological agents that target core clock proteins, timed delivery of existing drugs (chronotherapy), or lifestyle interventions—light exposure, sleep scheduling, and meal timing—that reinforce healthy rhythms systemically.
What comes next for research and treatment
The study opens multiple experimental directions: determining causal links between clock disruption and plaque accumulation, testing whether resetting glial clocks reverses pathology in animal models, and translating safe timing-based interventions into early clinical trials. Optimizing circadian biology may complement traditional strategies aimed at reducing protein aggregation or modulating immune responses.
Expert Insight
"This work reframes part of Alzheimer's as a disorder of timing as much as of molecular damage," says Dr. Lydia Farrow, a fictional neuroimmunologist specializing in circadian neuroscience. "If we can learn to tune the timing of gene activity in microglia and astrocytes, we may be able to preserve brain housekeeping long enough to slow or prevent downstream degeneration."
The study, published in Nature Neuroscience, builds on growing evidence that our internal clocks are tightly woven into brain health. By mapping which Alzheimer's risk genes rise and fall with the day, researchers have revealed a new layer of biology that could prove crucial for early intervention.
Source: sciencealert
Comments
DaNix
Hmm, is this causal or just correlation? amyloid could mess clocks, or clocks fail first... tough to know, needs more proof, quick thought
bioNix
wow, brain cleaning cells have clocks? mind blown. if true, timing drugs or sleep tweaks could actually matter. weird but hopeful


Leave a Comment