4 Minutes
New oxygen-breathing crystal unveiled
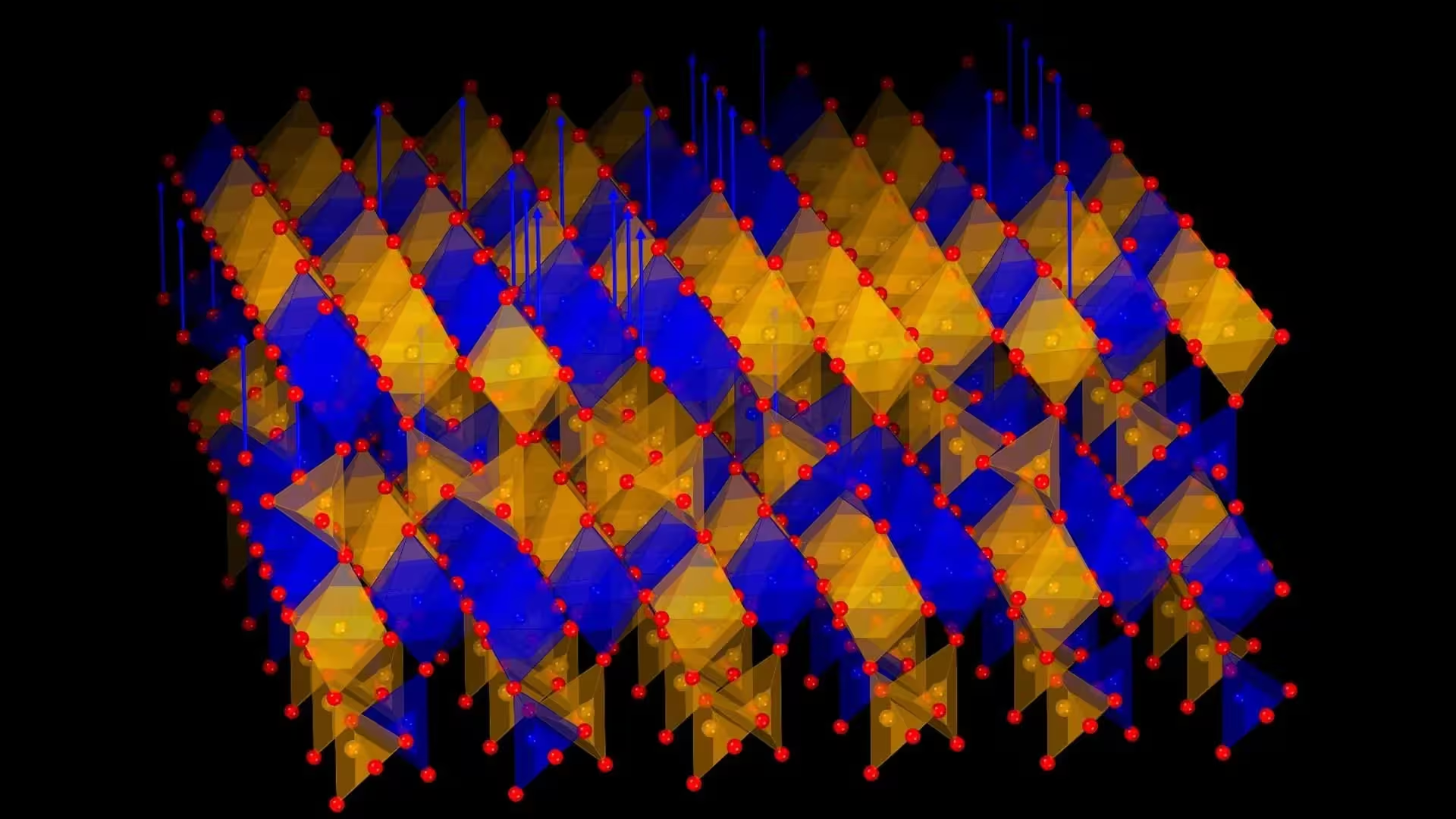
A multinational team from Korea and Japan has engineered a crystalline metal oxide that can repeatedly release and absorb oxygen at relatively low temperatures, a capability likened to "breathing." The material, composed of strontium, iron and cobalt, performs reversible oxygen exchange without structural collapse, opening pathways for clean energy devices and adaptive electronics. Credit: Prof. Hyoungjeen Jeen from Pusan National University, Korea
The discovery, led by Professor Hyoungjeen Jeen (Department of Physics, Pusan National University) with co-author Professor Hiromichi Ohta (Research Institute for Electronic Science, Hokkaido University), appears in Nature Communications (15 August 2025). The team demonstrated that the oxide releases oxygen when moderately heated in a simple gas environment and then reincorporates oxygen when conditions change — a fully reversible process that endures many cycles.
Scientific background and why oxygen control matters
Controlling oxygen content in solid materials is a central capability for several technologies. In solid oxide fuel cells (SOFCs), oxygen ion movement through metal oxides enables efficient conversion of hydrogen to electricity with low emissions. Thermal transistors — devices that modulate heat flow analogous to how electronic transistors switch electrical current — also rely on materials whose thermal conductivity changes with oxygen stoichiometry. Smart windows that dynamically change heat transmission can use similar oxygen-responsive mechanisms to adapt to weather and reduce building energy use.
What makes this crystal different?
Previous oxygen-exchange materials often demanded extreme temperatures or degraded after cycling. The new strontium–iron–cobalt oxide stands out because only the cobalt ions undergo reduction during oxygen release, and the rearrangement produces a new but stable crystal structure rather than destroying the lattice. Importantly, reintroducing oxygen restores the original crystal, confirming true reversibility — a key requirement for real-world applications.
Implications, applications and experimental highlights
Key implications include:
- Clean energy: Improved oxygen-handling materials can boost SOFC efficiency, durability and lower operating temperatures.
- Smart thermal devices: Materials that change thermal transport with oxygen content can enable thermal logic and adaptive insulation.
- Electronics and building materials: Reversible oxygen exchange could enable new nonvolatile memory concepts, sensors and energy-saving architectural coatings.
The research combined careful synthesis of the strontium–iron–cobalt oxide, controlled gas-environment heating experiments, and structural characterization that tracked ion valence and crystal-phase changes. The team emphasizes that the material remains intact through repeated cycles and operates under milder, more practical conditions than many prior candidates.
"It is like giving the crystal lungs; it can inhale and exhale oxygen on command," says Prof. Jeen, summarizing the discovery's novelty. Prof. Ohta adds, "This is a major step toward smart materials that can adjust themselves in real time." Their work highlights both fundamental insights into transition-metal redox chemistry and clear technological potential.
Expert Insight
Dr. Elena Park (independent materials scientist, fictional) comments: "Reversible oxygen exchange at low temperatures in a structurally robust oxide is rare. If scale-up preserves these properties, the material could lower operating temperatures for fuel cells and enable compact thermal switches. The next step is testing long-term cycling under application-relevant conditions and integrating thin-film versions into devices."
Conclusion
The strontium–iron–cobalt oxide reported by researchers in Korea and Japan demonstrates a reversible, low-temperature oxygen exchange that preserves crystal integrity — effectively giving the material controllable 'breathing' behavior. That combination of stability, reversibility and practical operating conditions makes it a promising candidate for fuel cells, thermal transistors, adaptive windows and other smart, energy-efficient technologies. Continued testing, scale-up, and device integration will determine how quickly this laboratory advance translates into commercial or industrial applications.
Source: sciencedaily

Leave a Comment