6 Minutes
Researchers have unveiled a novel refrigeration concept called ionocaloric cooling — a technique that uses charged particles to trigger phase changes and absorb heat. Promising significant efficiency gains and a much lower climate impact than today’s common refrigerants, ionocaloric systems could reshape how we cool buildings, food, and electronics.
How ionocaloric cooling actually works
Most modern refrigerators rely on vapor-compression cycles: a fluid evaporates to absorb heat, then is compressed and condensed to release it elsewhere. That approach is proven and efficient, but some of the gases used (notably hydrofluorocarbons, HFCs) have high global warming potential (GWP). Ionocaloric cooling takes a different route, using the physics of phase changes — and the movement of ions — to move heat.
Imagine a block of ice. Heat input melts the ice, and melting absorbs energy from the surroundings, cooling them. Ionocaloric systems force a similar phase change without needing large temperature swings: by shifting a material’s melting point through the introduction or movement of ions, the material can be made to absorb or release heat on demand.
In practice, the ionocaloric cycle uses an electric current to move charged particles through a fluid or solvent. Those ions alter intermolecular interactions and shift the temperature at which the substance changes phase. In lab tests at Lawrence Berkeley National Laboratory and UC Berkeley, researchers applied a small electrical bias to move ions and measured dramatic temperature changes.
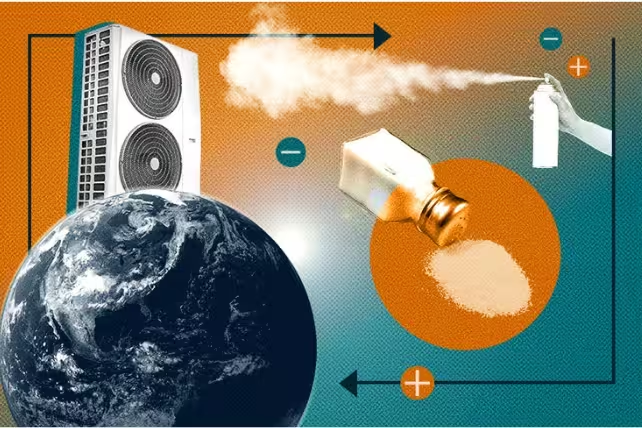
Illustration of the ionocaloric cycle concept. (Jenny Nuss/Berkeley Lab)
Key experiments and surprising performance
In experiments reported in Science, the team tested a salt made from sodium and iodine to melt ethylene carbonate, a common organic solvent also used in lithium-ion batteries. Ethylene carbonate can be produced from carbon dioxide, which opens the possibility that a working ionocaloric system could have net-zero or even negative GWP.
What stood out was the scale of the temperature change: the researchers recorded a roughly 25 °C (45 °F) shift after applying less than one volt. That result surpasses many caloric cooling approaches developed so far and hints at the strong potential for practical cooling and heating applications using small electrical inputs.
"The landscape of refrigerants is an unsolved problem," said Drew Lilley, a mechanical engineer at Lawrence Berkeley National Laboratory. "No one has successfully developed an alternative solution that makes stuff cold, works efficiently, is safe, and doesn't hurt the environment. We think the ionocaloric cycle has the potential to meet all those goals if realized appropriately."
The researchers also modeled thermodynamic performance and compared it to conventional refrigerants. Early results show that ionocaloric cycles could be competitive on energy efficiency while offering large reductions in environmental impact — provided materials and systems are engineered for real-world use.
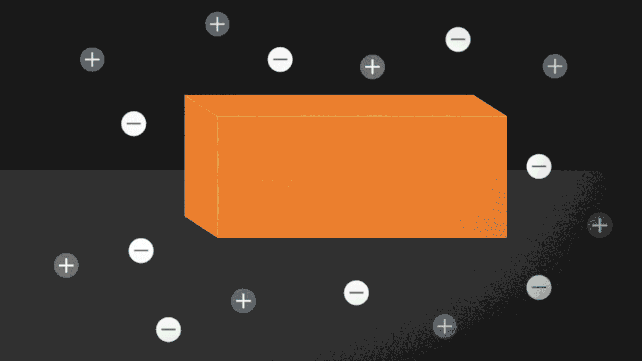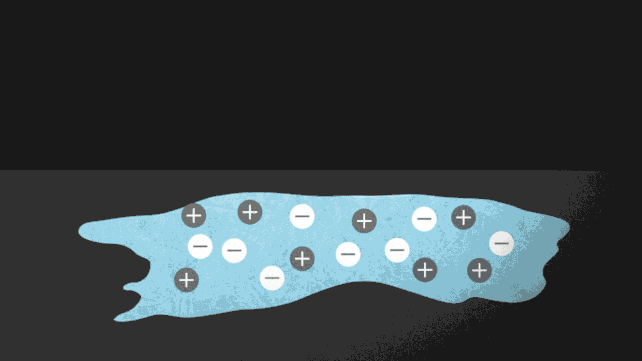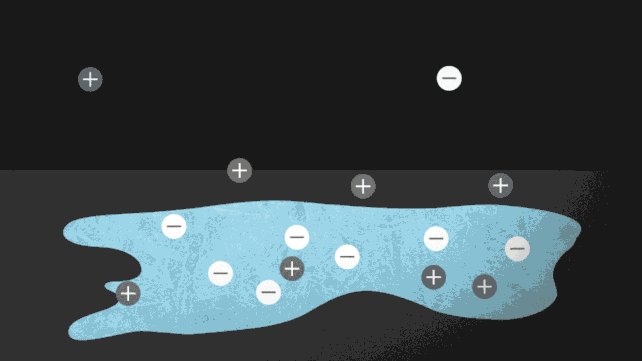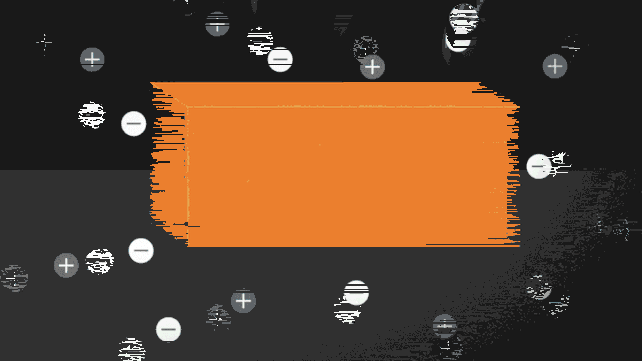
The ionocaloric cycle in action. (Jenny Nuss/Berkeley Lab)
Why this matters for climate policy and industry
Current refrigeration systems largely depend on HFCs, which many nations are phasing down under agreements such as the Kigali Amendment. That treaty commits signatories to cutting HFC production and consumption dramatically over the next 25 years. A practical ionocaloric technology could be an important part of that transition, offering a low-GWP alternative that still meets performance needs.
Beyond climate benefits, ionocaloric systems could bring other advantages: lower flammability and toxicity risks (depending on chosen materials), and the ability to run on electricity with fine control over temperature by modulating ion flow. Researchers are also exploring the reverse — using the cycle to deliver heat — which would broaden applications to heat pumps and building HVAC systems.
Engineering challenges and the road to commercialization
Laboratory results are promising, but scaling a new thermodynamic cycle into reliable, cost-effective equipment is a complex engineering problem. Teams must balance three main factors: the GWP of working fluids, overall energy efficiency, and equipment cost. Early tests suggest ionocaloric approaches can be favorable across all three, but that must hold up under long-term cycling, real-world loads, and industry safety standards.
Ongoing work includes screening different salts and solvents to find the best combinations for stability, capacity, and recyclability. In 2025, an international team published an efficient version of the cycle using nitrate-based salts that were recycled using electric fields and selective membranes — exactly the kind of materials innovation the Berkeley group expected to follow their initial proof-of-concept.
"There are three things we're trying to balance: the GWP of the refrigerant, energy efficiency, and the cost of the equipment itself," said Ravi Prasher, another mechanical engineer at Berkeley Lab. "From the first try, our data looks very promising on all three of these aspects."
Expert Insight
"Ionocaloric cooling is an elegant blending of electrochemistry and thermodynamics," says Dr. Lena Park, a climate-tech systems engineer who has advised several clean-cooling startups. "The physics is sound, and the early temperature swings are impressive. But the real test will be materials durability and how these systems integrate with existing appliances and HVAC infrastructure. If those hurdles are cleared, ionocaloric devices could dramatically reduce the climate footprint of cooling worldwide."
Researchers are now focused on material optimization, long-duration cycling tests, and engineering prototypes that can be evaluated in realistic conditions. If successful, ionocaloric cooling may move rapidly from lab benches to factories, supermarkets, data centers, and homes — a rare example of a new thermodynamic cycle with immediate climate relevance.
Source: sciencealert
Comments
labcore
is this even true? lab tests look flashy, but what about stability after 10k cycles, membrane fouling, cost of salts, real COP in the field. sounds promising but skeptical.
atomwave
wow didn't expect a 25°C swing with under 1V! if that scales, cooling could change fast. curious about long-term cycles, corrosion, recycle of salts tho, fingers crossed


Leave a Comment