4 Minutes
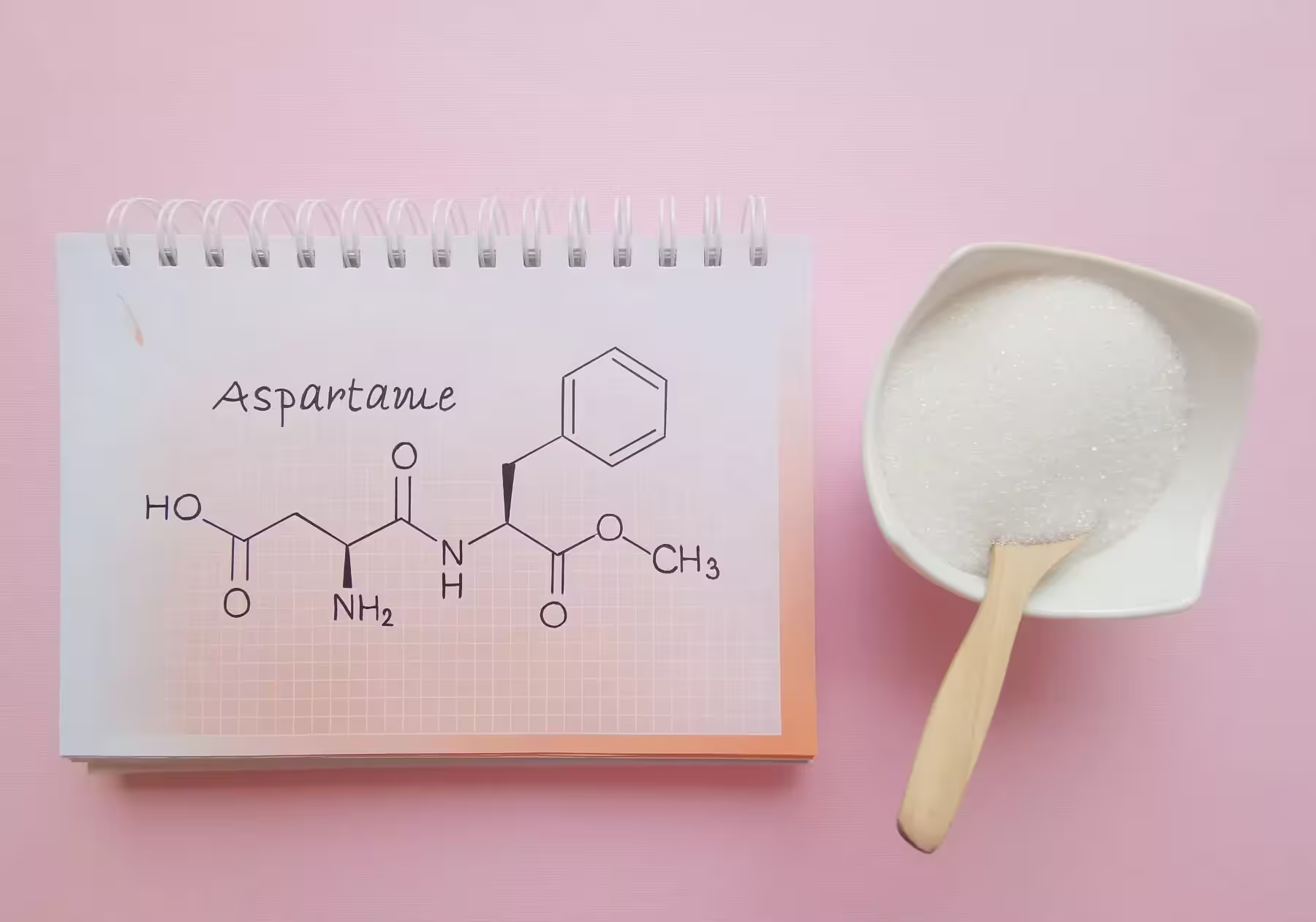
A new long-term animal study from Spain raises fresh questions about the safety of aspartame, a popular low-calorie sweetener used in diet sodas, chewing gum and many reduced-sugar products. Researchers report measurable changes in heart structure and cognitive performance in mice exposed to low doses over an extended period — findings that could prompt regulatory agencies to reassess current safety limits.
How the experiment was designed and what researchers measured
The study, led by investigators at a collaborative biomedicine research center in San Sebastián and published in Biomedicine & Pharmacotherapy, followed mice for one year with continuous exposure to aspartame at 7 mg per kilogram of body weight. That daily dose is roughly one-sixth of the maximum intake currently recommended for humans by WHO, the European Medicines Agency and the U.S. FDA.
Scientists combined functional and molecular imaging, cognitive tests, and a range of physiological measurements to build a comprehensive picture of chronic aspartame exposure. The result is the first detailed long-term animal study specifically designed to probe chronic effects on heart and brain function.
Key findings: reduced fat, altered hearts, and cognitive decline
One surprising metabolic result: treated mice showed about a 20% reduction in body fat. But that apparent benefit came with worrying pathophysiological changes. Researchers observed signs consistent with altered cardiac morphology — including indicators of mild cardiac hypertrophy alongside reductions in mid‑wall muscle thickness — and significant drops in cardiac output. Measured output from the left and right ventricles fell by approximately 26% and 20%, respectively.
Behavioral and cognitive testing also revealed declines in performance consistent with compromised brain function. The team reported accompanying molecular and imaging signals in brain tissue that align with the observed cognitive and behavioral changes.
Interpreting the results: caution and context
Authors of the paper emphasize that these results demonstrate even permitted aspartame doses can affect vital organ function in mice, and they call for a review of human safety limits. However, the International Sweeteners Association (ISA) urged caution about directly extrapolating mouse data to humans. Fundamental physiological differences — including metabolism, lifespan, cardiac physiology and how the human brain uses energy — complicate direct translation of effects seen in rodents.
Past human research has also shown that low‑calorie sweeteners alone are not a guaranteed method for weight loss, and some observed effects in rodents could reflect interactions with normal aging processes rather than a simple cause–effect relationship.
Why it matters and next steps for research
This study adds to a complex body of literature on artificial sweeteners. For consumers, it doesn't immediately prove harm in humans, but it highlights the need for more long-term clinical research and possibly updated safety assessments from regulators. Future human trials and mechanistic studies will be needed to determine whether the cardiac and cognitive signals seen in mice occur in people, at what doses, and via which molecular pathways.
Practical takeaways for readers
- Low doses of aspartame produced measurable heart and brain changes in mice after prolonged exposure.
- Animal results are an important warning signal but do not prove the same outcomes will appear in humans.
- Regulators and independent researchers should re-evaluate long-term exposure studies and, if warranted, reconsider acceptable daily intake recommendations.
- Consumers who are concerned can moderate intake of diet sodas and processed products containing aspartame while awaiting further human-focused research.

Leave a Comment