5 Minutes
A promising laboratory study shows that a drug candidate called P7C3-A20 can reverse cognitive decline in mouse models with advanced Alzheimer's-like pathology. By restoring cellular energy chemistry and reducing inflammation, researchers say the damaged brain can recover important functions even without clearing hallmark protein aggregates.
How a single compound targets brain energy
Scientists at Case Western Reserve University and collaborators tested P7C3-A20, a neuroprotective compound that helps restore balance to nicotinamide adenine dinucleotide (NAD+). NAD+ is a central metabolic molecule: cells use it to convert nutrients into usable energy and to support DNA repair and protein maintenance. Low NAD+ is linked to aging and a range of neurodegenerative conditions.
In this study, mice with advanced Alzheimer’s-like symptoms received daily injections of P7C3-A20 for six months. The treatment returned NAD+ levels to normal, reduced markers of inflammation and DNA damage, and—critically—stopped brain cell deterioration. That biochemical recovery translated into measurable gains in learning and memory in both mouse lines tested.
Two different Alzheimer's models, one common outcome
The team deliberately used two genetically distinct mouse models, each representing one of Alzheimer's two hallmark pathologies: amyloid-beta plaques and tau tangles. These aggregated proteins are widely seen in human Alzheimer's brains, but their exact role in causing cognitive decline remains complex and debated.
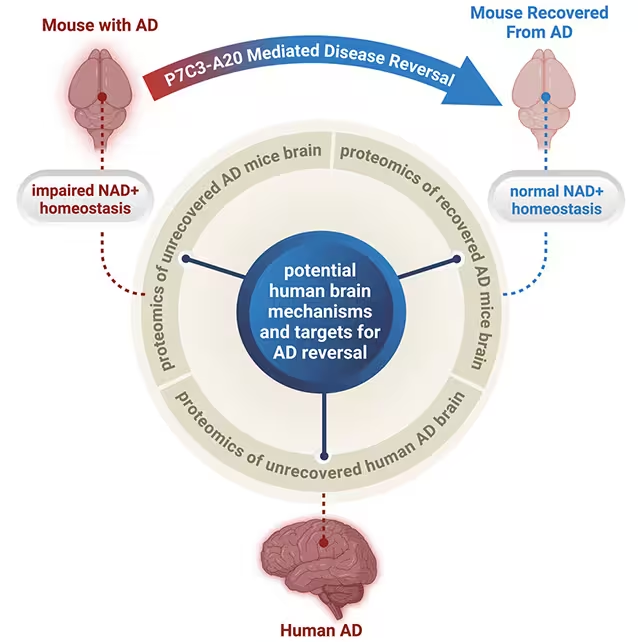
Remarkably, P7C3-A20 improved behavior and neuropathology markers in both models without clearing plaques or tangles. "Restoring the brain's energy balance achieved pathological and functional recovery in both lines of mice with advanced Alzheimer's," says neuroscientist and psychiatrist Andrew Pieper. The result suggests that if neurons regain sufficient metabolic resilience, they may better tolerate—or compensate for—protein aggregates.
What the findings mean for Alzheimer's research
Restoring NAD+ appears to be the critical mechanism behind the recovery. Prior animal studies have shown NAD+ boosters can improve outcomes after brain injury; this work extends those findings to chronic neurodegeneration. The Cell Reports Medicine paper reported that markers for inflammation and DNA damage fell in treated mice, indicating cells regained the capacity to maintain normal function.
Still, the team urges caution. Translating results from mice to humans is notoriously difficult: dosing, timing, side effects, and long-term safety must be proven in carefully controlled clinical trials. Researchers also note that excessive NAD+ activity has been linked to increased cancer risk in some contexts, so any human therapy would need precise calibration and monitoring.
Why this doesn't mean an instant cure
Alzheimer's is a multifactorial disease involving genetics, protein aggregation, inflammation, vascular health and more. P7C3-A20's effect does not eliminate plaques or tangles, but it may allow neurons to function despite them—an important conceptual shift. "Seeing this effect in two very different animal models, each driven by different genetic causes, strengthens the new idea that recovery from advanced disease might be possible in people with AD when the brain's NAD+ balance is restored," Pieper says.
Potential paths forward: trials and safety checks
Next steps include additional preclinical work to confirm dosing windows, long-term effects, and safety across ages and genetic backgrounds. If those studies are encouraging, carefully designed human trials could begin—likely starting with early-phase safety studies in volunteers and patients with mild-to-moderate Alzheimer's.
Researchers will also explore whether NAD+ restoration is most effective alone or as part of a combination treatment that addresses inflammation, vascular health, and protein aggregation simultaneously. Given Alzheimer's complexity, a multi-pronged therapeutic strategy seems plausible.
Expert Insight
Dr. Lena Morales, a fictional but realistic senior neuroscientist at a major research university, comments: "This study shifts how we think about resilience in the aging brain. Rather than removing every pathological hallmark, boosting cellular metabolism and repair systems could let neurons recover functionality—at least for a time. It's an encouraging, pragmatic approach that complements other therapeutic strategies."
While much work remains, this study offers a hopeful, testable avenue: recalibrate the brain's energy economy and you may unlock recovery even in advanced disease stages. The research was published in Cell Reports Medicine (2025), and the lead team emphasizes careful, methodical follow-up before any human application is considered.
Source: sciencealert
Comments
Tomas
is this even true? mouse brains arent tiny humans. good sign but dont forget dosing, safety and longterm effects, could backfire
bioNix
wait, seriously? if neurons can recover without clearing plaques that's huge!! Hope trials are careful tho, fingers crossed

Leave a Comment