2 Minutes
Researchers have developed a cell-engineering method that dramatically lowers the amount of viral vector needed to produce CAR-equipped induced natural killer (iNK) cells. The advance promises to cut costs and improve scalability for CAR-based immunotherapies while preserving potent anti-tumor activity in preclinical models.
How the new technique slashes viral-vector demand
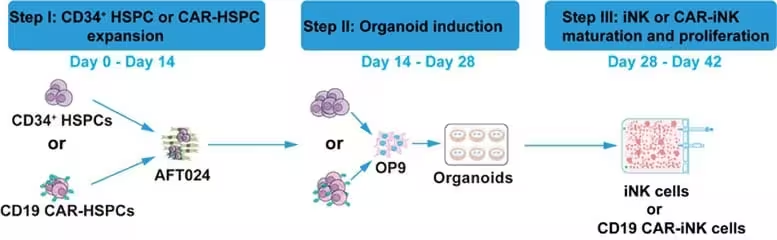
Traditional CAR engineering of mature NK cells typically requires substantial amounts of viral vector to deliver the receptor gene. The new approach, applied during the induction of iNK cells, reduced vector input by staggeringly large factors compared with standard protocols — roughly on the order of 1:140,000 by the sixth week and as low as about 1:600,000 by the seventh week of culture. In plain terms, researchers achieved comparable CAR expression using only a tiny fraction of the viral material normally required.
Functional tests: tumor control in leukemia models
Lower vector usage would be meaningless without preserved function. In multiple preclinical assays, both unmodified iNK cells and CAR-modified iNK (CAR-iNK) cells showed strong cytotoxicity against cancer cells. In mouse models—both cell line-derived xenografts (CDX) and patient-derived xenografts (PDX) of human B-cell acute lymphoblastic leukemia (B-ALL)—CD19-directed CAR-iNK cells slowed tumor growth and extended survival compared with controls.
Why this matters for cell therapy development
Viral vectors are among the most expensive components of CAR cell manufacturing. Cutting the required viral input by orders of magnitude has two immediate benefits: lower production costs and reduced biosafety burden during scale-up. That combination can accelerate translational work and make next-generation NK-cell therapies more accessible to clinical trials.
Implications and next steps
While the results are preclinical, they indicate a pathway to more efficient CAR engineering that retains therapeutic potency. Future work will need to confirm durability, safety, and performance in larger animal studies and early human trials. If those studies succeed, clinicians may soon have a cost-effective, off-the-shelf NK-cell platform to target CD19 and other tumor antigens.
Source: scitechdaily

Leave a Comment